ChemicalBook > CAS DataBase List > Mesoridazine Besylate
Mesoridazine Besylate
Mesoridazine Besylate
- CAS No.32672-69-8
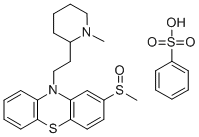
- Chemical Name:Mesoridazine Besylate
- CBNumber:CB2295822
- Molecular Formula:C27H32N2O4S3
- Formula Weight:544.75
- MOL File:32672-69-8.mol
Mesoridazine Besylate Property
- Melting point 174-175 °C
- storage temp. 2-8°C
- solubility DMSO: 10 mg/mL
- form solid
- color white
- Stability Hygroscopic
- FDA UNII T4G2I958J2
- UNSPSC Code 41116107
- NACRES NA.24
Safety
-
Symbol(GHS)

- Signal wordWarning
- Hazard statements H302
- Precautionary statements P301+P312+P330
Mesoridazine Besylate Chemical Properties,Usage,Production
- Originator Serentil,Sandoz,US,1970
- Uses Mesoridazine (M225755) Besylate is an antipsychotic.
- Uses antpsychotic
- Definition ChEBI: The benzenesulfonate salt of mesoridazine prepared using equimolar amounts of mesoridazine and benzenesulfonic acid.
-
Manufacturing Process
10.0 g of 3-methylmercapto phenothiazine and 17.5 cc of acetic acid
anhydride are refluxed for 8 hours from an oil bath maintained at a
temperature of 180°C. After concentration of the solution the residue is
crystallized from ethanol. The pure 3-methylmercapto-10-acetyl phenothiazine
melts at 89° to 91°C. For the purpose of oxidation 5.0 g of 3-
methylmercapto-10-acetyl phenothiazine are dissolved in 50 cc of ethanol,
refluxed from an oil bath maintained at 120°C and 1.6 cc of a 40% hydrogen
peroxide solution are then added dropwise in the course of 30 minutes.
Heating is continued for another 5 hours and the reaction mixture is concentrated after 50 cc of water have been added. The residue is taken up in 40 cc of benzene and the benzene layer washed with 10 cc of water. After having been concentrated, the residue, crude 3-methylsulfinyl-10-acetyl phenothiazine, is dissolved in 55 cc of a 90% methanol solution for splitting off the acetyl group and, after 2.9 g of potassium carbonate have been added, it is boiled for 2 hours under reflux on an oil bath kept at a temperature of 120°C. After concentration, the residue is taken up in 50 cc of chloroform, the chloroform layer is washed with a total of 25 cc of water, dried over potassium carbonate, filtered and concentrated. After twice crystallizing the residue, each time from 50 cc of ethanol, analytically pure 3-methylsulfinyl phenothiazine (MP 193° to 195°C) is obtained.
A mixture of 10.0 g of 3-methylsulfinyl phenothiazine (MP 193° to 195°C), 6.1 g of finely powdered sodium hydroxide and 125 cc of toluene is boiled for 1 hour under reflux with a water separator on an oil bath kept at a temperature of 150°C, while the mixture is stirred. Without interrupting the boil a solution of 7.0 g of 2-(N-methyl-piperidyl-2')-1-chloroethane (BP 84°C/10 mm Hg) in 10 cc of toluene is added dropwise in the course of 1 hour, after which boiling is continued for another 3 hours. When the reaction mixture has cooled it is first washed with 25 cc of water three times and then extracted with 75 cc of a 15% aqueous tartaric acid solution. The tartaric acid extract is shaken out with 25 cc of benzene, 20 cc of concentrated caustic soda are added until the phenolphthalein reaction is alkaline, and the separated oily base is taken up in a total of 150 cc of benzene.
After having been washed with 50 cc of water the benzene layer is dried over potassium carbonate, filtered, allowed to stand over 10 g of alumina for about 1? hours for partial decolorization, filtered again and concentrated under reduced pressure. The oily base which remains as a residue is directly converted into the tartrate. A solution cooled to 0°C, of 6.50 g of the free base in 100 cc of acetic acid ethyl ester is thoroughly shaken and poured into an ice cold solution of 2.66 g of tartaric acid in 410 cc of acetic acid ethyl ester. The precipitated, analytically pure, tartrate of 3-methylsulfinyl-10-[2'-Nmethyl-piperidyl-2')-ethyl-l']-phenothiazine melts at 115° to 120°C (foam formation) and sinters above 80°C. The base is reacted with benzene sulfonic acid in a suitable solvent to give the besylate. - brand name Serentil (Novartis).
- Therapeutic Function Tranquilizer
- General Description Mesoridazine besylate,10-[2-(methyl-2-piperidyl)ethyl]-2-(methylsulfinyl)phenothiazinemonobenzenesulfonate (Serentil), shares manyproperties with thioridazine. However, no pigmentaryretinopathy has been reported.
- Biological Activity Mesoridazine benzenesulfonate is a D2 dopamine receptor antagonist; antipsychotic.
Mesoridazine Besylate Preparation Products And Raw materials
Raw materials
Preparation Products
Global(47)Suppliers
-
Supplier:
Shaanxi Dideu Medichem Co. Ltd
- Tel:+86-029-81138252<br/>+86-18789408387
- Email:1057@dideu.com
- Country:China
- ProdList:3922
- Advantage:58
-
Supplier:
TargetMol Chemicals Inc.
- Tel:+1-781-999-5354<br/>+1-00000000000
- Email:marketing@targetmol.com
- Country:United States
- ProdList:32159
- Advantage:58
-
Supplier:
TargetMol Chemicals Inc.
- Tel:
- Email:support@targetmol.com
- Country:United States
- ProdList:38630
- Advantage:58
-
Supplier:
Aladdin Scientific
- Tel:
- Email:tp@aladdinsci.com
- Country:United States
- ProdList:57505
- Advantage:58
- Supplier: Sinopharm Chemical Reagent Co,Ltd.
- Tel:86-21-63210123
- Email:sj_scrc@sinopharm.com
- Country:China
- ProdList:9815
- Advantage:79
- Supplier: Shanghai TaoSu Biochemical Technology Co., Ltd.
- Tel:021-33632979
- Email:info@tsbiochem.com
- Country:China
- ProdList:8065
- Advantage:58
- Supplier: Shanghai YuanYe Biotechnology Co., Ltd.
- Tel:021-61312847;<br/>18021002903
- Email:3008007409@qq.com
- Country:China
- ProdList:71829
- Advantage:60
- Supplier: Beijing Solarbio Science & Tecnology Co., Ltd.
- Tel:010-50973186<br/>4009686088
- Email:3193328036@qq.com
- Country:China
- ProdList:18338
- Advantage:68
- Supplier: Aikon International Limited
- Tel:025-58859352<br/>18068836627
- Email:yftan@aikonchem.com
- Country:China
- ProdList:15494
- Advantage:58
- Supplier: Angel Pharmatech, Ltd.
- Tel: 17317130613
- Email:3358272972@qq.com
- Country:China
- ProdList:3260
- Advantage:58
32672-69-8, Mesoridazine BesylateRelated Search:
- SERENTIL
- 抑制剂
- 药靶配体
- C27H32N2O4S3
- C21H26N2OS2C6H6SO3
- 苯磺酸美索达嗪,10 MM DMSO 溶液
- MESORIDAZINE BENZENESULFONATE|||SERENTIL|||苯磺酸美索达嗪
- 甲磺酸美索达嗪
- 盐酸硫利达嗪EP杂质B苯磺酸盐
- 10-[2-(1-甲基-2-哌啶基)乙基]-2-(甲基亚磺酰基)-10H-苯并噻嗪
- 美索达斯汀
- 盐酸甲硫哒嗪杂质B(EP)
- 苯磺酸美索达嗪 USP标准品
- 苯磺酸美索达嗪
- 32672-69-8
- Mesoridazine Besylate, 10 mM in DMSO
- Mesoridazine Besilate (10-[2-[(2RS)-1-Methylpiperidin-2-yl]ethyl]-2-(methylsufhinyl)-10H-phenothiazi
- Thioridazine EP Impurity B Besylate (Mesoridazine Besylate)
- Mesoridazine benzenesulfonate|||Serentil
- Thioridazine Hydrochloride Impurity B as Besilate
- Thioridazine Hydrochloride EP Impurity B as Besilate
- 10-(2-(1-Methylpiperidin-2-yl)ethyl)-2-(methylsulfinyl)-10H-phenothiazine benzenesulfonate
- Mesoridazine Besilate (10-[2-[(2RS)
- Mesoridazine Besylate (1393005)
- Mesoridazine Besylate (250 mg)
- 10-[2-(1-METHYL-2-PIPERIDINYL)ETHYL]-2-(METHYLSULFINYL)-10H-PHENOTHIAZINE
- MESORIDAZINE BESYLATE
- MESORIDAZINE BENZENESULFONATE
- ylsulfinyl)ethyl)-10h-phenothiazine(1:1)
- nc123
- benzenesulfonicacid,compd.with10-(2-(1-methyl-2-piperidinyl)ethyl)-2-(meth
- SERENTIL