ChemicalBook > CAS DataBase List > Shikonin
Shikonin
Shikonin
- CAS No.517-89-5
- Chemical Name:Shikonin
- CBNumber:CB2251044
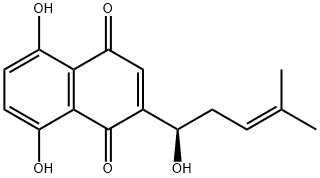
- Molecular Formula:C16H16O5
- Formula Weight:288.3
- MOL File:517-89-5.mol
Shikonin Property
- Melting point 148℃
- Boiling point 567.4±50.0 °C(Predicted)
- Density 1.373±0.06 g/cm3(Predicted)
- storage temp. -20°C
- solubility DMSO : ≥ 31 mg/mL (107.53 mM);
- pka 7.34±0.20(Predicted)
- form Red crystalline solid.
- color Brown
- LogP 4.350 (est)
- CAS DataBase Reference 517-89-5(CAS DataBase Reference)
- FDA UNII 3IK6592UBW
- UNSPSC Code 41116107
- NACRES NA.77
Safety
- Risk Statements :20/21/22-50/53-20
- Safety Statements :26-36/37/39-61
- RIDADR :3077
- HS Code :29146990
- Hazardous Substances Data :517-89-5(Hazardous Substances Data)
-
Symbol(GHS)

- Signal wordWarning
- Hazard statements H315-H319
- Precautionary statements P264-P280-P302+P352-P337+P313-P305+P351+P338-P362+P364-P332+P313
Shikonin Price
More Price(1)
- Brand: Sigma-Aldrich(India)
- Product number: 565850
- Product name : Shikonin - CAS 517-89-5 - Calbiochem
- Purity: A naphthoquinone found in the Chinese herb Shiunko.
- Packaging: 10MG
- Price: ₹13100
- Updated: 2022/06/14
- Buy: Buy
Shikonin Chemical Properties,Usage,Production
-
Plant extract
The purple-colored roots of red gromwell (Lithos-permum erythrorhizon), also known as “zicao” in Chinese, “jichi” in Korean, and “murasaki” in Japanese, have been used as part of traditional medicines, as adyestuff, and in cosmetics across many cultures for centuries. The responsible bioactive and pigmented compounds, shikonin—or its enantiomer, alkannin—and dozens of other acylated shikonin/alkannin derivatives, are synthesized in the root periderm of L. erythrorhizon and several other Boraginaceae species.Shikonins/alkannins are deposited into the rhizosphere where they function in plant-microbe interactions and interfere with the growth of competing plants (allelopathy), roles suggested having contributed to the invasion success of species like Echium plantagineum. The presence of alkannins was also reported in Plagiobothrysarizonicus leaves, though the physiological and/or ecological significance of their presence in aerial tissues is unclear. Shikonin is produced in the roots of Lithospermum erythrorhizon. Fig. Intact roots of L. erythrorhizon producing shikonin. Fig b. Hairy root culture of L. erythrorhizon producing shikonin[1].

-
Description
Shikonin could be found in the plant of Lithospermum erythrorhizon Sieb. et. Zucc
and Arnebia euchroma (Royle) Johnst. It could also be produced using plant cell
culture techniques. Shikonin is recorded in British Pharmacopoeia as a reference
compound.
Zicao, a traditional Chinese medicine firstly recorded in Shen Nong’s Herbal Classic, has long been used medically in history . Chinese Pharmacopoeia (Edition 2015) recorded the root of Arnebia euchroma (Royle) Johnst and Arnebia guttata Bge as Zicao, which is mainly used for inflammatory diseases such as macular eruptions, measles, sore throat, carbuncles, and burns. - Chemical Properties Purple-brown crystals with a needle-like shape, melting at 147 ° C, with a rotativity of αD20 = +135 ° (in benzene). It dissolves readily in phenethyl ether, acetone, chloroform, methanol, ethanol, glycerol, animal and vegetable oils, and alkaline aqueous solutions, but is insoluble in water. Its color changes depending on the pH value: at pH 4-6, it turns red; at pH 8, it becomes purple; and at pH 10-12, it appears blue. It exhibits excellent resistance to light, heat, and oxidation, but is reactive to reducing agents, causing the formation of dark purple when exposed to iron ions. Additionally, Shikonin has certain antibacterial effect.
- History Kuroda and Majima firstly identified acetyl shikonin from L. erythrorhizon in 1922 , followed by the discovery of other shikonin derivatives, including shikonin. The chemical structure of shikonin was not precisely identified till 1936 for its high physicochemical similarity with naphthazarin . There have been about 500 publications on shikonin up to now. Great attention has also been paid on the biosynthesis of shikonin and its derivatives, and an increasing number of shikonin derivatives have been designed and synthesized for exploring their antitumor effect. There are two types of derivatives: one is modifications of 1′-OH with parent nucleus naphthazarin remained and the other is modifications of both 1′-OH and parent nucleus naphthazarin, as shown in Fig. 3c, d .
- Uses Shikonin has been used as a red dye for centuries and is reported to possess medicinal properties such as antibacterial, anti-inflammatory and antitumor activities. It occurs as an acetyl derivative in the Japanese shikone, Lithospermum erythrorhizon, another member of the Boraginaceae family. It is the (R)-optical isomer of alkannin. Tissue cultures of L. erythrorhizon are used in Japan to manufacture shikonin mainly for cosmetic use. Both alkannin and shikonin are mordant dyes producing violet to gray colors on fabrics. In Japan, shikonin was used to dye fabrics a color known as Tokyo Violet. Shikalkin the racemate, has been synthesized.
- Definition ChEBI: Shikonin is a hydroxy-1,4-naphthoquinone. It is a naphthoquinone derivative with angiogenesis inhibitor properties.
- General Description Shikonin is a naturally occurring naphthoquinine isolated from the dried root of L. erythrorhizon, an herb used in traditional Chinese medicine. It increases glucose uptake by adipocytes and myocytes and inhibits the activity of phosphatase and tensin homolog (PTEN; IC50 = 2.7 μM). It inhibits glycolysis in cancer cells by inhibiting tumor-specific pyruvate kinase M2 (IC50 = 0.3 μM). Shikonin induces cell death consistent with necroptosis in MCF-7 and HEK293 cancer cell lines. It inhibits leukocyte migration, downregulates chemokine receptor expression, and inhibits HIV-1 replication at nanomolar concentrations. Shikonin exhibits anti-inflammatory activity, reducing joint swelling and cartilage destruction in a mouse model of collagen-induced arthritis.
-
Pharmacology
Shikonin possesses anti-inflammatory, antioxidant, antiviral, cardiovascular protective,and antitumor activities.
Shikonin reduces inflammation by inhibiting the biosynthesis of leukotrienes and 5-hydroxyeicosatetraenoic acid and thus reduces synthesis of inflammation-related active molecules, which selectively block chemokine binding to CC chemokine receptor 1 .
Shikonin shows free radical scavenging and antioxidant (especially toward superoxide anion and DPPH) activities. It significantly inhibits autoxidation caused by β-carotene and linoleic acid . Its anti-HCV effects have been reported recently with an EC50 at 25 ng/mL, which is lower than that of ribavirin (2.6 μg/mL) .
Recent studies also reveal shikonin possesses cardiovascular protective effects. Shikonin inhibits the activity of TNF-α promoter, revealing its transcriptional antagonism to pro-inflammatory cytokine . Shikonin also shows antitumor potentials by inducing apoptosis and/or necrosis, inhibiting DNA topoisomerase activity and angiogenesis, and regulating proliferative signaling pathways (including MAPK, VEGF, and PTKs ). In addition, shikonin circumvents cancer drug resistance by induction of necroptotic cell death . - Clinical Use Shikonin and its derivatives have not been approved for clinical use yet. Studies are confined to cellular and animal experiments. Its original plant Zicao has a long history of medical use both orally and externally in China. Various dosage forms of Zicao, including tablets, injections, oils, creams, tinctures, plastics, and pastes, have been developed for different clinical applications especially in dermatology, gynecology, pediatrics, ophthalmology, and otorhinolaryngology. Among them, puccoon oil and lithospermum cream are the most widely used forms.
- storage Store at -20°C
- References [1] Robert P Auber. “Hybrid de novo genome assembly of red gromwell (Lithospermum erythrorhizon) reveals evolutionary insight into shikonin biosynthesis.” ACS Biomaterials Science & Engineering (2020): 82.
Shikonin Preparation Products And Raw materials
Raw materials
Preparation Products
Global(348)Suppliers
-
Supplier:
Hebei Mujin Biotechnology Co.,Ltd
- Tel:+86 13288715578<br/>+8613288715578
- Email:sales@hbmojin.com
- Country:China
- ProdList:12809
- Advantage:58
-
Supplier:
Wuhan Haorong Biotechnology Co.,ltd
- Tel:+86-18565342920;<br/>+8618565342920
- Email:sales@chembj.net
- Country:China
- ProdList:305
- Advantage:58
-
Supplier:
Hebei Zhuanglai Chemical Trading Co Ltd
- Tel:+86-16264648883<br/>+86-16264648883
- Email:niki@zlchemi.com
- Country:China
- ProdList:7245
- Advantage:58
-
Supplier:
Henan Tianfu Chemical Co.,Ltd.
- Tel:+86-0371-55170693<br/>+86-19937530512
- Email:info@tianfuchem.com
- Country:China
- ProdList:21628
- Advantage:55
-
Supplier:
Shanghai Zheyan Biotech Co., Ltd.
- Tel:18017610038
- Email:zheyansh@163.com
- Country:CHINA
- ProdList:3619
- Advantage:58
-
Supplier:
Chengdu Greenpure Biopharma CO.,Ltd
- Tel:18283602253;<br/>+8618283602253
- Email:jancyzheng@gcgreenpure.com
- Country:China
- ProdList:953
- Advantage:58
-
Supplier:
career henan chemical co
- Tel:+86-0371-86658258<br/>+8613203830695
- Email:sales@coreychem.com
- Country:China
- ProdList:29858
- Advantage:58
-
Supplier:
Chengdu GLP biotechnology Co Ltd
- Tel:028-87075086<br/>13350802083
- Email:scglp@glp-china.com
- Country:CHINA
- ProdList:1824
- Advantage:58
-
Supplier:
Chengdu Biopurify Phytochemicals Ltd.
- Tel:+86-28-82633860;<br/>+8618080483897
- Email:sales@biopurify.com
- Country:China
- ProdList:3772
- Advantage:58
-
Supplier:
Hubei Jusheng Technology Co.,Ltd.
- Tel:18871490254
- Email:linda@hubeijusheng.com
- Country:CHINA
- ProdList:28172
- Advantage:58
Related articles
517-89-5, ShikoninRelated Search:
- Anthraquinones, Hydroquinones and Quinones
- standardized herbal extract
- reference standards from Chinese medicinal herbs (TCM).
- phytochemical
- pharmaceutical intermediate
- chemical reagent
- Inhibitors
- 蒽醌类
- 日化
- 抑制剂
- 精品科研试剂
- 其它原料及中间体
- 其他
- 高端化学
- 保质期
- 试剂
- 高含量单体
- 化学试剂
- 单体香料
- 高含量产品
- 色素
- 原料药
- 香料香精
- 含量产品
- 医药原料
- 标准品,对照品
- 医药、农药及染料中间体
- 医药原料药
- 其它天然产物
- 分析试剂-标准品
- 日化原料
- 标准品 -中药标准品
- 原料
- 对照品-中药对照品
- 医用原料
- 标准品-中药标准品
- 植物提取
- 细胞生物学试剂
- 其它植物提取物
- 食品和饮料标准品
- 着色剂
- 小分子抑制剂,天然产物
- 小分子抑制剂
- 植提标准品
- 对照品
- 标准品
- 植物提取物
- 分析标准品
- 中药对照品
- 食用色素与护色剂
- 食用色素(着色剂)
- 食品添加剂
- 中草药成分
- C16H16O5
- (±)-SHIKONIN
- 紫草素,10 MM DMSO 溶液
- 紫草素/醌·萘醌·蒽醌
- 紫草素/HPLC/右旋