ChemicalBook > CAS DataBase List > TROVAFLOXACIN
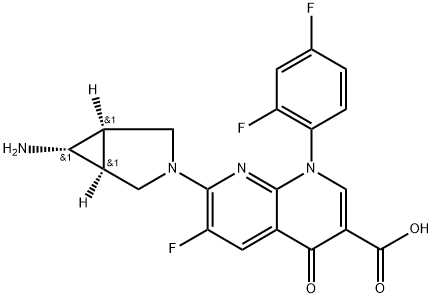
TROVAFLOXACIN
TROVAFLOXACIN
- CAS No.147059-72-1
- Chemical Name:TROVAFLOXACIN
- CBNumber:CB1777404
- Molecular Formula:C20H15F3N4O3
- Formula Weight:416.36
- MOL File:147059-72-1.mol
TROVAFLOXACIN Property
- Melting point >195oC (dec.)
- Boiling point 630.5±55.0 °C(Predicted)
- Density 1.612±0.06 g/cm3(Predicted)
- storage temp. -20°C Freezer, Under inert atmosphere
- solubility Acetonitrile (Slightly), DMSO (Slightly)
- form Solid
- pka 5.80±0.70(Predicted)
- color Pale Beige to Light Beige
- CAS DataBase Reference 147059-72-1
- FDA UNII 9F388J00UK
- ATC code J01MA13
-
Symbol(GHS)

- Signal wordWarning
- Hazard statements H302-H315-H319-H335
- Precautionary statements P261-P305+P351+P338
TROVAFLOXACIN Chemical Properties,Usage,Production
- Originator Trovan,Pfizer,USA
- Uses Antibacterial.
- Uses Trovafloxacin is a fluroquinolone antibiotic, recently identified as an inhibitor of pannexin-1 (Panx1) channels. Trovafloxacin attenuates neuroinflammation and improves outcome after traumatic brain injury in mice.
- Definition ChEBI: A 1,8-naphthyridine derivative that is 4-oxo-1,4-dihydro-1,8-naphthyridine-3-carboxylic acid bearing additional 2,4-difluorophenyl, fluoro and 6-amino-3-azabicyclo[3.1.0]hex-3-yl substituents at positions 1, 6 and 7 respectively. A broad-spectrum antibiot c that was withdrawn from the market due to risk of liver failure.
-
Manufacturing Process
N-Benzylmaleimide (500 g, 2.67 mole), 90% bromonitromethane (831 g, 5.34
mole), powdered molecular sieves 200 mesh (2020 g) and toluene (12 dm3)
were stirred under nitrogen at -10°C. 1,2-Dimethyl-1,4,5,6-
tetrahydropyrimidine (616 g, 5.49 mole) was added slowly over about 3 h
maintaining the reaction temperature at <-8°C throughout the addition. After
completion of the addition, the reaction mixture was stirred for 1.5 h at 25°C,
filtered under a nitrogen atmosphere in a sealed pressure filter to remove
sieves and resulting tar, and the sieves were washed with toluene (2 L). The
combined filtrates were washed with 2 N dilute hydrochloric acid (3 times 750
cm3), treated with carbon (50 g) at 70°C, 1 h filtered, concentrated, and
triturated with 2-propanol (about 4 dm3) to obtain crystals of the (1α,5α,6α)-
3-N-benzyl-6-nitro-2,4-dioxo-3-azabicyclo[3.1.0]hexane (223 g, 34%) melting
point 116°-118°C.
Tetrahydrofuran (350 cm3), sodium borohydride (14.1 g) and (1α,5α,6α)-3-N_x0002_benzyl-6-nitro-2,4-dioxo-3-azabicyclo[3.1.0]hexane (35.0 g, mmol) obtained above were stirred under nitrogen for 0.25 h and then treated dropwise with boron trifluoride-THF complex containing 21.5% BF3 (44.9 cm3) so that the exotherm was controlled to <40°C. After addition was completed, the reaction mixture was stirred for 3 h at 40°C, quenched slowly with water/THF 1:1 (70 cm3) to avoid excessive foaming, and stirred for 0.5 h at 50°C to ensure that the quench of unreacted diborane generated in situ was completed. The quench formed a salt slurry which was filtered and washed with THF (140 cm3); the combined filtrate was partially concentrated, diluted with water (350 cm3) and further concentrated to remove most of the THF, and extracted with ethyl acetate (140 cm3). The resulting ethyl acetate solution was concentrated to afford the (1α,5α,6α)-3-N-benzyl-6-nitro-3-azabicyclo[3.1.0]hexane as a clear oil (30.6 g, 97%).
(1α,5α,6α)-3-N-Benzyl-6-nitro-3-azabicyclo[3.1.0]hexane (30.9 g, 142 mmol) obtained above, 2-propanol (310 cm3), water (30 cm3), and 10% Pd on carbon, 50% water content (12.3 g) were hydrogenated at 50 psi and 50°C for 18-24 h in a Parr shaker. The Pd catalyst was filtered off, and the resulting pale yellow filtrate was azeotropically distilled at constant volume to remove water. The resulting solution was treated with triethylamine (46 g, 456 mmol) and heated to reflux. Benzaldehyde (15.0 g, 141 mmol) was added dropwise over 15 min. The reaction mixture was heated at reflux for 4 h to form (1α,5α,6α)-6-benzylidenylamino-3-azabicyclo[3.1.0]hexane in situ. The resulting orange solution was cooled to 40°-50°C, and ethyl 7-chloro-1-(2,4-difluorophenyl)-6-fluoro-1,4-dihydro-4-oxo-1,8-naphthyridine-3-carboxylic acid (42.45 g, 111 mmol; see United Kingdom Patent Publication No. GB 2,191,776) and triethylamine (13.1 g, 130 mmol) were added. The resulting slurry was heated at reflux for 16-18 h, cooled to 20°C and stirred for 5 h. The slurry was filtered, and the compound was isolated as a white solid (75.5% yield based on (1α,5α,6α)-3-N-benzyl-6-nitro-2,4-dioxo-3- azabicyclo[3.1.0]hexane; 96.6% based on ethyl 7-chloro-1-(2,4- difluorophenyl)-6-fluoro-1,4-dihydro-4-oxo-1,8-naphthyridine-3-carboxylic acid). The ethyl (1α,5α,6α)-7-(6-benzylidenylamino-3-azabicyclo[3.1.0]hex- 3yl)-1-(2,4-difluorophenyl)-6-fluoro-1,4-dihydro-4-oxo-1,8-naphthyridine-3- carboxylate was recrystallized from acetonitrile, melting point 148°-155°C decomp.
Tetrahydrofuran (250 cm3), ethyl (1α,5α,6α)-7-(6-benzylidenylamino-3- azabicyclo[3.1.0]hex-3-yl)-1-(2,4-difluorophenyl)-6-fluoro-1,4-dihydro-4-oxo- 1,8-naphthyridine-3-carboxylate (25.05 g, 47 mmol) obtained above, and water (250 cm3) were treated with 97% methanesulfonic acid (13.3 g, 138 mmol) and heated to reflux for 24 h. The resulting solution was cooled to 45°C, treated with activated carbon (2.5 g) for 1 h and filtered. The resulting filtrate was concentrated under vacuum to approximately 25% of its original volume to provide a white crystal slurry, cooled to 15°-25°C, granulated for 4 h and filtered to yield the trovafloxacin methanesulfonate salt (mesylate) (16.86 g, 70.0%). Melting point 253°-256°C decomp. - brand name Trovan (Pfizer).
- Therapeutic Function Antibacterial
TROVAFLOXACIN Preparation Products And Raw materials
Raw materials
Preparation Products
Global(71)Suppliers
-
Supplier:
TargetMol Chemicals Inc.
- Tel:+1-781-999-5354<br/>+1-00000000000
- Email:marketing@targetmol.com
- Country:United States
- ProdList:32159
- Advantage:58
-
Supplier:
Career Henan Chemica Co
- Tel:+86-0371-86658258<br/>+8613203830695
- Email:laboratory@coreychem.com
- Country:China
- ProdList:30231
- Advantage:58
-
Supplier:
Dideu Industries Group Limited
- Tel:+86-29-89586680<br/>+86-15129568250
- Email:1026@dideu.com
- Country:China
- ProdList:22859
- Advantage:58
-
Supplier:
AFINE CHEMICALS LIMITED
- Tel:+86-0571-85134551
- Email:sales@afinechem.com
- Country:China
- ProdList:15350
- Advantage:58
-
Supplier:
Zhejiang J&C Biological Technology Co.,Limited
- Tel:+1-2135480471<br/>+1-2135480471
- Email:sales@sarms4muscle.com
- Country:China
- ProdList:10473
- Advantage:58
-
Supplier:
InvivoChem
- Tel:+1-708-310-1919<br/>+1-13798911105
- Email:sales@invivochem.cn
- Country:United States
- ProdList:6391
- Advantage:58
-
Supplier:
Wuhan Topule Biopharmaceutical Co., Ltd
- Tel: +8618327326525
- Email:masar@topule.com
- Country:China
- ProdList:8467
- Advantage:58
-
Supplier:
LEAPCHEM CO., LTD.
- Tel:+86-852-30606658
- Email:market18@leapchem.com
- Country:China
- ProdList:43340
- Advantage:58
-
Supplier:
Shanghai Acmec Biochemical Technology Co., Ltd.
- Tel:+86-18621343501;<br/>+undefined18621343501
- Email:product@acmec-e.com
- Country:China
- ProdList:33338
- Advantage:58
-
Supplier:
Jilin Chinese Academy of Sciences-yanshen Technology
- Tel: +undefined18143011203
- Email:info@chemextension.com
- Country:China
- ProdList:42056
- Advantage:58
147059-72-1, TROVAFLOXACINRelated Search:
- Clinafloxacin Sparfloxacin Gemifioxacin Gatifloxacin Moxifloxacin Balofloxacin Pazufloxacin Tosufloxacin Sparfloxacin Alatrofloxacin 3-Fluoro-5-methylpyridine 3-FLUORO-5-FORMYLPYRIDINE 6-PIPERIDIN-1-YLNICOTINALDEHYDE 3-azabicyclo[3.1.0]hexane 2-Amino-5-fluoropyridine 6-(1-PYRROLIDINYL)NICOTINALDEHYDE 2-Amino-3-fluoropyridine 2-Amino-3-acetylpyridine
- API
- 现货
- 精细化工原料
- 合成抗菌药
- 药物
- 喹诺酮类药物
- 抗病源性微生物药
- C20H15F3N4O3
- 曲伐沙星杂质
- 曲氟沙星
- 聚苯乙烯磺酸钙
- 曲伐沙星,曲氟沙星
- (1Α,5Α,6Α)-7-(6-氨基-3-氮杂二环[3.1.0]己-3-基)-1-(2,4-二氟苯基)-6-氟-1,4-二氢-4-氧-1,8-萘啶-3-羧酸
- 特伐沙星
- 曲伐沙星
- 147059-72-1
- rel-7-((1R,5S,6s)-6-Amino-3-azabicyclo[3.1.0]hexan-3-yl)-1-(2,4-difluorophenyl)-6-fluoro-4-oxo-1,4-dihydro-1,8-naphthyridine-3-carboxylic acid
- NF-κB,inhibit,Topoisomerase,PANX1,DNA,gyrase,Antibiotic,LPS,pro-inflammatory,hepatotoxicity,topoisomerase-IV,Inhibitor,pneumococci,Trovafloxacin,Bacterial,broad-spectrum,TNF
- Trovafloxacin (CP-99219)
- TROVAFLOXACIN USP/EP/BP
- 1,8-Naphthyridine-3-carboxylic acid, 7-[(1α,5α,6α)-6-amino-3-azabicyclo[3.1.0]hex-3-yl]-1-(2,4-difluorophenyl)-6-fluoro-1,4-dihydro-4-oxo-
- 7-[(1R,5S)-6-azanyl-3-azabicyclo[3.1.0]hexan-3-yl]-1-(2,4-difluorophenyl)-6-fluoro-4-oxo-1,8-naphthyridine-3-carboxylic acid
- 7-[(1R,5S)-6-amino-3-azabicyclo[3.1.0]hexan-3-yl]-1-(2,4-difluorophenyl)-6-fluoro-4-oxo-1,8-naphthyridine-3-carboxylic acid
- 7-[(1R,5S)-6-amino-3-azabicyclo[3.1.0]hexan-3-yl]-1-(2,4-difluorophenyl)-6-fluoro-4-keto-1,8-naphthyridine-3-carboxylic acid
- Trovan:CP-99219-27
- CP-99219
- (1α,5α,6α)-7-(6-Amino-3-azabi-cyclo[3.1.0]hex-3-y1)-1-(2,4-difluomphenyl)-6-fluom-1,4-dihydro-4-oxo-1,8-naphthyridine-3-carboxylic acid
- 1,8-Naphthyridine-3-carboxylic acid, 7-(1.alpha.,5.alpha.,6.alpha.)-6-amino-3-azabicyclo3.1.0hex-3-yl-1-(2,4-difluorophenyl)-6-fluoro-1,4-dihydro-4-oxo-
- TROVAFLOXACIN