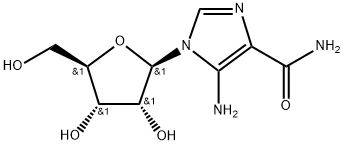
ChemicalBook > CAS DataBase List > AICAR
AICAR
AICAR
- CAS No.2627-69-2
- Chemical Name:AICAR
- CBNumber:CB1675294
- Molecular Formula:C9H14N4O5
- Formula Weight:258.23
- MOL File:2627-69-2.mol
AICAR Property
- Melting point 214-215 °C
- Boiling point 726.3±60.0 °C(Predicted)
- Density 2.06±0.1 g/cm3(Predicted)
- storage temp. -20°C
- solubility H2O: >10 mg/mL
- form powder
- pka 13.27±0.70(Predicted)
- color tan
- biological source rabbit
- Water Solubility Soluble in DMSO at 2mg/ml. Soluble in water or ethanol at less than 1mg/ml
- λmax 265nm(NaOH)(lit.)
- Merck 14,16
- Specific Activity 255-345nmol/min·mg
- Stability Stable for 2 years from date of purchase as supplied. Solutions in DMSO or distilled water may be stored at -20°C for up to 3 months.
- InChIKey RTRQQBHATOEIAF-UUOKFMHZSA-N
- CAS DataBase Reference 2627-69-2(CAS DataBase Reference)
- FDA UNII F0X88YW0YK
- NCI Drug Dictionary acadesine
- ATC code C01EB13
- EPA Substance Registry System 1H-Imidazole-4-carboxamide, 5-amino-1-.beta.-D-ribofuranosyl- (2627-69-2)
- UNSPSC Code 12352203
- NACRES NA.77
Safety
- Hazard Codes :Xi,T
- Risk Statements :36/37/38-61
- Safety Statements :26-36-45-53
- WGK Germany :3
- TSCA :Yes
- HS Code :29349990
-
Symbol(GHS)


- Signal wordDanger
- Hazard statements H315-H319-H360D
- Precautionary statements P202-P264-P280-P302+P352-P305+P351+P338-P308+P313
AICAR Price
More Price(11)
- Brand: Sigma-Aldrich(India)
- Product number: SRP5004
- Product name : AMPK (α2/β2/γ2), active, His tagged human
- Purity: PRECISIO? Kinase, recombinant, expressed in baculovirus infected Sf9 cells, ≥70% (SDS-PAGE), buffered aqueous glycerol solution
- Packaging: 5μG
- Price: ₹84415.5
- Updated: 2022/06/14
- Buy: Buy
- Brand: Sigma-Aldrich(India)
- Product number: SRP5003
- Product name : AMPK (α2/β2/γ1), active, His tagged human
- Purity: PRECISIO? Kinase, recombinant, expressed in baculovirus infected Sf9 cells, ≥70% (SDS-PAGE), buffered aqueous glycerol solution
- Packaging: 5μG
- Price: ₹84415.5
- Updated: 2022/06/14
- Buy: Buy
- Brand: Sigma-Aldrich(India)
- Product number: SAB4502332
- Product name : Anti-AMPK β1 antibody produced in rabbit
- Purity: affinity isolated antibody
- Packaging: 100μG
- Price: ₹50560.5
- Updated: 2022/06/14
- Buy: Buy
- Brand: Sigma-Aldrich(India)
- Product number: SAB1406293
- Product name : Anti-PRKAA1 antibody produced in mouse
- Purity: purified immunoglobulin, buffered aqueous solution
- Packaging: 50μG
- Price: ₹50671.5
- Updated: 2022/06/14
- Buy: Buy
- Brand: Sigma-Aldrich(India)
- Product number: SAB1306034
- Product name : ANTI-AMPK ALPHA2(C-TERMINAL) antibody produced in rabbit
- Purity: IgG fraction of antiserum, buffered aqueous solution
- Packaging: 400μL
- Price: ₹47796.6
- Updated: 2022/06/14
- Buy: Buy
AICAR Chemical Properties,Usage,Production
-
adenosine regulating agents
Acadesine is the prototype of a new class of compounds termed adenosine regulating agents. Acadesine is a purine nucleosid analogue that enters the myocyte and is immediately phosphorylated to ZMP (AICA ribotide), which is further metabolised to Inosine mono-phosphate (an intermediate in the synthesis ofadenosine triphosphate (ATP) and guanosine triphos-phate).
Claims that acadesine may serve as a substrate for ATP synthesis and result in repletion of myocardial ATP were supported by some studies and refuted by others. Because acadesine may be a precursor in the synthesis of myocardial ATP it was proposed as a possible agent of myocardial protection during ischaemia, particularly because myocardial ATP depletion has been linked to cell death. - Description AICAR (2627-69-2) activates AMP-activated protein kinase (AMPK). Promotes ligand-independent activation of the insulin receptor.1 Promotes skeletal muscle autophagy via activation of FoxO3a.2 Controls smooth muscle cell hyperproliferation in vascular disease.3 ?Induces osteogenic differentiation in mesenchymal stem cells.4 Inhibits proinflammatory response in glial cells.5 Cell permeable.
- Chemical Properties Solid
- Originator AICA,BIOMOL
- Uses glucose uptake stimulant; AMPK activator
- Uses AICAR is a nucleoside analogue that is able to enter nucleoside pools and is able to significantly increase levels of adenosine during periods of ATP breakdown. Adenosine-regulating agents (ARAs) hav e been recognized for therapeutic potential in myocardial ischemia. Cardioprotective.
- Uses 5-Aminoimidazole-4-carboxamide 1-beta-D-ribofuranoside is used as a cell permeable activator of AMP-activated protein kinase (AMPK), a metabolic master regulator that is activated in times of reduced energy availability (high cellular AMP:ATP ratios) and serves to inhibit anabolic processes. In vivo, pharmacologic activation of AMPK with AICAR mimics exercise and triggers insulin-independent glucose uptake by skeletal muscle.
- Definition ChEBI: A 1-ribosylimidazolecarboxamide in which the carboxamide group is situated at position 4 of the imidazole ring, which is further substituted at position 5 by an amino group. A purine nucleoside analogue and activator of AMP-activated protein kinase, it is is used for the treatment of acute lymphoblastic leukemia and is reported to have cardioprotective effects.
-
Manufacturing Process
Adenosine 3', 5'-cyclic phosphate N'-oxide (76.0 g, 0.200 mole) as the dihydrate was dissolved in a solution of 400 ml DMSO and 31.0 g (0.204
mole) 1,5-diazabicyclo[5.4.0]undec-5-ene. The solution was cooled to 15°C
and 40 ml methyl iodide was added with stirring at room temperature. After
30 min, the mixture had gelled; 1.5 L ethanol was added and the solid was
thoroughly homogenized by vigorous stirring. The solid was filtered, and the
resulting paste was resuspended in 2 L ethanol and homogenized. The product
was again filtered, washed with ethanol and ether, and dried, giving 80.4 g of
1-methoxyadenosine 3',5'-cyclic phosphate suitable for further transformation
(recrystallization from aqueous methanol with ether).
A solution of 30.0 g 1-methoxyadenosine 3',5'-cyclic phosphate (81.5 mmole), 20.0 g NaHCO3 (238 mmole), and 300 ml H2O was refluxed 45 mm. The pH of the solution was adjusted to 2.5 with Dowex 50x8 (H)+ while warm, and a water pump vacuum was applied to mixture to remove CO2. The pH was readjusted to 9-10 with NaOH, and the resin was removed by filtration. The solution was passed onto a column containing 400 ml Dowex 1x2 (formate, 100-200 mesh), and the column was washed well with water. The column was eluted with a gradient of 4 L water in the mixing chamber and 4 L 4 N formic acid in the reservoir. The first major product, coming after about 2 L eluate, was 5-amino-N-methoxy-1-β-D-ribofuranosylimidazole-4-carboxamidine 3',5'- cyclic phosphate, giving 5.4 g (19%) after evaporation of the solvent and trituration of the residue with ethanol (recrystallization from water). A solution of 5.0 g (14.3 mmoles) 5-amino-N-methoxy-1-β-Dribofuranosylimidazole- 4-carboxamidine 3',5'-cyclic phosphate in 200 ml H2 preheated to 60°C and containing approximately 5.0 g moist sponge nickel catalyst, was shaken with 2-3 atm. H2 at 60°C for 2 h. The filtered solution was evaporated to dryness to give 3.75 g of 5-amino-1-β-Dribofuranosylimidazole- 4-carboxamidine 3',5'-cyclic phosphate (82%), (recrystallization from water).
A mixture of 4.0 g (12.5 mmole) 5-amino-1-β-D-ribofuranosylimidazole-4- carboxamidine 3',5'-cyclic phosphate and 100 ml conc. NH4OH was heated in a bomb at 100°C for 16 h, then cooled and evaporated in vacuum. The residue was taken up in 100 ml H2O and applied to a 2.5x20 cm column of Dowex 1x2 (formate form, 100-200 mesh). After washing well with H2O the column was eluted with a gradient of 1 L H2O in the mixing chamber and 1 L 3 N formic acid in the reservoir. Fractions containing the product, appearing near the end of the elution, were evaporated. Trituration of the residue with EtOH gave 2.90 g (68%) of 5-amino-1-β-D-ribofuranosylimidazole-4- carboxamide 3',5'-cyclic phosphate.
The 5-amino-1-β-D-ribofuranosylimidazole-4-carboxamide may be produced by hydrolysis of 5-amino-1-β-D-ribofuranosylimidazole-4-carboxamide 3',5'- cyclic phosphate with NaOH. - Therapeutic Function Cardiotonic, Platelet aggregation inhibitor
- Biological Functions The first direct AMPK activator, 5-aminoimidazole-4-carboxamide riboside (AICAR), is an adenosine analog taken up into cells by adenosine transporters and phosphorylated by adenosine kinase, thus generating the AMP-mimetic, AICAR monophosphate (ZMP). Similarly to cellular AMP, ZMP binds to site 3 on the AMPKγ subunit. Although ZMP is a much less potent AMPK activator than AMP in cell-free systems, AICAR directly activates AMPK in most cells because ZMP can accumulate to millimolar concentrations in cells. AICAR is able to activate many other AMP-dependent enzymes, such as fructose-1,6-bisphosphatase.
- General Description Adenosine monophosphate?protein kinase (AMPK) activator 5-aminoimidazole-4-carboxamide?ribonucleotide?(AICAR)?modulates cellular energy. It regulates lipid and glucose metabolism, pro-inflammatory responses,?cytokine production,?cell proliferation and apoptosis. AICAR improves ischemia or reperfusion injury and kidney fibrosis in rats. It provides protection against acute tubular necrosis.
- Biological Activity Cell-permeable, allosteric activator of AMP-activated protein kinase (AMPK). Augments proliferation, differentiation and mineralization of osteoblastic MC3T3-EI cells and attenuates psychosine-induced expression of proinflammatory cytokines and iNOS in astrocytes.
- Biochem/physiol Actions AICAR is a cell permeable activator of AMP-activated protein kinase (AMPK), a metabolic master regulator that is activated in times of reduced energy availability (high cellular AMP:ATP ratios) and serves to inhibit anabolic processes. In vivo, pharmacologic activation of AMPK with AICAR mimics exercise and triggers insulin-independent glucose uptake by skeletal muscle.
- Side effects AICAR is an AMPK (adenosine 5′-monophosphate (AMP)-activated protein kinase) activator that has been shown to have significant endurance-enhancing effects and is banned for use as a doping agent by the World Anti-Doping Agency. Overactivation of AMPK, or activation in the wrong tissues, can lead to serious side effects, including neurodegeneration or preventing cell division. Accumulation of naturally occurring AICAR in the body has also been linked to metabolic disorders in humans. However, AICAR has not been approved for human treatment and cannot be used as a drug. Therefore, its safety has yet to be verified.
- storage -20°C
- Clinical claims and research AICAR has proved in vivo anti-tumor effects in xenograft models. It is currently under clinical trial phase I/II to treat patients with chronic lymphocytic leukemia and has shown good safety and tolerability properties, although some side effects have been reported.
- References 1) Chopra et al. (2012), Phosphorylation of the insulin receptor by AMP-activated protein kinase (AMPK) promotes ligand-independent activation of the insulin signalling pathway in rodent muscle; Diabetologia, 55 783 2) Sanchez et al. (2012), AMPK promotes skeletal muscle autophagy through activation of forkhead FoxO3a and interaction with Ulk1; J. Cell Biochem., 113 695 3) Ferri et al. (2012), AMP-activated protein kinase and the control of smooth muscle cell hyperproliferation in vascular disease; Vascul. Pharmacol., 56 9 4) Wu et al. (2011) AICAR, a small chemical molecule, primes osteogenic differentiation of adult mesenchymal stem cells; Int. J. Artif. Organs, 34 1128 5) Giri et al. (2004) 5-aminoimidazole-4-carboxamide-1-beta-4-ribofuranoside inhibits proinflammatory response in glial cells: a possible role of AMP-activated protein kinase; J. Neurosci., 24 479
AICAR Preparation Products And Raw materials
Raw materials
Preparation Products
Global(364)Suppliers
-
Supplier:
Changzhou Xuanming Pharmaceutical Technology Co., Ltd.
- Tel:+86-519-85525359<br/>+86-15995072465
- Email:sales@xuanmingchem.com
- Country:China
- ProdList:914
- Advantage:58
-
Supplier:
Anko Intermational Trade Limited
- Tel:+86-18073326374<br/>+86-18073326374
- Email:sales@goodpeptides.com
- Country:China
- ProdList:56
- Advantage:58
-
Supplier:
Wuhan Ruichi Technology Co., Ltd
- Tel: +8613545065237
- Email:admin@whrchem.com
- Country:China
- ProdList:153
- Advantage:58
-
Supplier:
Shanghai Affida new material science and technology center
- Tel: +undefined15081010295
- Email:admin@oudaxin.com
- Country:China
- ProdList:114
- Advantage:58
-
Supplier:
Huaian Banting Trading Co., Ltd.
- Tel: +8618875735705
- Email:sales3@bantingpeptide.com
- Country:China
- ProdList:57
- Advantage:58
-
Supplier:
American HealthyMorph LLC
- Tel: +8613363867578
- Email:nancyyuan133@163.com
- Country:China
- ProdList:168
- Advantage:58
-
Supplier:
Hebei Chuanghai Biotechnology Co., Ltd
- Tel: +8615531157085
- Email:abby@chuanghaibio.com
- Country:China
- ProdList:8808
- Advantage:58
-
Supplier:
Hebei Mujin Biotechnology Co.,Ltd
- Tel:+86 13288715578<br/>+8613288715578
- Email:sales@hbmojin.com
- Country:China
- ProdList:12809
- Advantage:58
-
Supplier:
Hebei Chuanghai Biotechnology Co,.LTD
- Tel: +86-13131129325
- Email:sales1@chuanghaibio.com
- Country:China
- ProdList:5868
- Advantage:58
-
Supplier:
Wuhan Han Sheng New Material Technology Co.,Ltd
- Tel: +8617798174412
- Email:admin01@hsnm.com.cn
- Country:China
- ProdList:2097
- Advantage:58
Related articles
2627-69-2, AICARRelated Search:
- AICAR (phosphate) Inosine Impurity 1 L-Inosine Inosine 2-CHLOROINOSINE XANTHOSINE 2',3',5'-TRI-O-ACETYLINOSINE IDP 2'-Deoxyguanosine-5'-diphosphate trisodium salt 2',3'-O-Isopropylideneinosine XANTHOSINE 5'-MONOPHOSPHATE DISODIUM SALT Disodium 5'-Inosinate 3'-GMP DISODIUM SALT Guanosine GUANOSINE 3':5'-CYCLIC MONOPHOSPHATE SODIUM SALT Succino-AICAR 5-Amino-4-imidazolecarboxamide INOSINE-3',5'-CYCLIC PHOSPHATE
- SARMS
- Nucleotides
- Carbohydrates & Derivatives
- Bases & Related Reagents
- Protein Kinase
- mTOR
- Akt
- PI3K
- PI3K/Akt/mTOR
- Cardiovascular APIs
- ARASINE
- 抑制剂
- 药靶配体
- 合成有机化合物配体
- 实验室产品
- 精细化工原料
- 出口SARM系列
- 科研原料
- 标准品
- 试剂盒-Elisa试剂盒
- 试剂盒-细胞分析试剂盒
- 库存
- 医药、农药及染料中间体
- 化工原料
- 其他生化试剂
- 细胞生物学试剂
- 保健、减肥原料药
- 化工
- 药品
- 抗肿瘤及免疫抑制剂
- 抗肿瘤类
- 抗血小板聚集药
- 医药原料
- 医药中间体
- 小分子抑制剂
- 医药原料药
- Cell Signaling and Neuroscience
- Cell Biology
- BioChemical
- AMP-Activated Protein Kinase (AMPK)
- Serine/Threonine Kinase Activators
- Kinase/Phosphatase Biology
- C9H14N4O5
- 627-69-2
- AP标记的腺苷单磷酸活化蛋白激酶Γ2抗体
- HRP标记的腺苷单磷酸活化蛋白激酶Γ2抗体
- BIOTIN标记的腺苷单磷酸活化蛋白激酶Γ2抗体
- AICAR(ACADESINE,阿卡地新) |CAS 2627-69-2
- 阿卡地新,10 MM DMSO 溶液
- AICAR (ACADESINE/AICA RIBOSIDE),AMPK活化剂
- AICAR (ACADESINE/AICA RIBOSIDE) (MM/ML),AMP-ACTIVATED蛋白KINASE活化剂
- AICAR (ACADESINE/AICA RIBOSIDE) (AQUEOUS SOLUTION),蛋白KINASE活化剂
- AICAR 化学试剂
- 阿卡德辛
- 阿卡地新 (AMPK激活剂)
- 5-AMINOIMIDAZOLE-4-CARBOXAMIDE-1-BETA-RIBOSIDE,AICAR 阿卡地新ACADESINE, AMP-ACTIVATED PROTEIN KINASE ACTIVATOR
- 阿卡地新,AMP活化蛋白激酶激活剂
- 5-氨基咪唑-4-甲酰胺-1-B-D-呋喃核糖苷 25MG