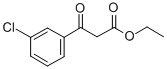
ChemicalBook > CAS DataBase List > Ethyl (3-chlorobenzoyl)acetate
Ethyl (3-chlorobenzoyl)acetate
Ethyl (3-chlorobenzoyl)acetate
- CAS No.33167-21-4
- Chemical Name:Ethyl (3-chlorobenzoyl)acetate
- CBNumber:CB1359370
- Molecular Formula:C11H11ClO3
- Formula Weight:226.66
- MOL File:33167-21-4.mol
Ethyl (3-chlorobenzoyl)acetate Property
- Boiling point 257-258 °C (lit.)
- Density 1.213 g/mL at 25 °C (lit.)
- refractive index n
20/D 1.5460(lit.) - Flash point >230 °F
- storage temp. Sealed in dry,Room Temperature
- pka 9.91±0.46(Predicted)
- CAS DataBase Reference 33167-21-4(CAS DataBase Reference)
- UNSPSC Code 12352100
- NACRES NA.22
Safety
- WGK Germany :3
-
Symbol(GHS)

- Signal wordWarning
- Hazard statements H319-H335-H315
- Precautionary statements P264-P280-P302+P352-P321-P332+P313-P362-P264-P280-P305+P351+P338-P337+P313P
Ethyl (3-chlorobenzoyl)acetate Price
More Price(2)
- Brand: Sigma-Aldrich(India)
- Product number: 559288
- Product name : Ethyl (3-chlorobenzoyl)acetate
- Purity: ≥97%
- Packaging: 1G
- Price: ₹8724.95
- Updated: 2022/06/14
- Buy: Buy
- Brand: Sigma-Aldrich(India)
- Product number: 559288
- Product name : Ethyl (3-chlorobenzoyl)acetate
- Purity: ≥97%
- Packaging: 5G
- Price: ₹43083.5
- Updated: 2022/06/14
- Buy: Buy
Ethyl (3-chlorobenzoyl)acetate Chemical Properties,Usage,Production
- Uses Reagent / reactant involved in:• ;Oxidative cross-coupling via dioxygen activation with indoles1• ;Chlorination and hydrosilylation for synthesis of α-hydroxy β-amino acid derivatives2• ;Precursor for substrates for chiral Lewis base-catalyzed stereoselective reduction with trichlorosilane and water3• ;Intramolecular cyclization for synthesis of dihydrofurans4• ;Rate acceleration of Michael reactions5• ;Cerium ammonium nitrate-mediated oxidative coupling6
-
Uses
Reagent / reactant involved in:
- Oxidative cross-coupling via dioxygen activation with indoles
- Chlorination and hydrosilylation for synthesis of α-hydroxy β-amino acid derivatives
- Precursor for substrates for chiral Lewis base-catalyzed stereoselective reduction with trichlorosilane and water
- Intramolecular cyclization for synthesis of dihydrofurans
- Rate acceleration of Michael reactions
- Cerium ammonium nitrate-mediated oxidative coupling
-
Synthesis
141.6 g of diethyl carbonate was dissolved in 1000 mL of a 1:1 ethanol-tetrahydrofuran (THF) solution, and 12.4 g of catalyst 1 Amberlite IRA-400 was added while stirring. The mixture was then heated up to 45 °C. In a separate container, 154.5 g of m-chloroacetophenone was dissolved in 1000 mL of the above-mentioned ethanol-THF mixed solvent. The addition of m-chloroacetophenone was completed within 1 hour, and stirring was continued for 6 hours. The mixture was then filtered. The filtrate was combined and the majority of the solvent was evaporated. 1500 mL of water was added to the remaining solution while stirring. The solution was then subjected to CH2Cl2 extraction, followed by washing with saturated saline until the solution reached a neutral pH. The resulting solution was dried with anhydrous MgSO4, filtered, and the solvent was evaporated. Finally, the resulting residue was distilled under reduced pressure to obtain a colorless oily liquid known as Ethyl (3-chlorobenzoyl)acetate.
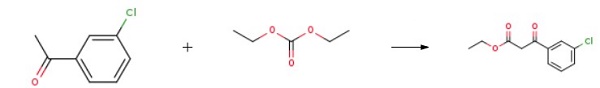
Ethyl (3-chlorobenzoyl)acetate Preparation Products And Raw materials
Raw materials
Preparation Products
Global(93)Suppliers
-
Supplier:
Shanghai Gubang New Materail Technology Co. LTD
- Tel:21-51317962<br/>+8618721305404
- Email:16728589@qq.com
- Country:CHINA
- ProdList:272
- Advantage:58
-
Supplier:
Alchem Pharmtech,Inc.
- Tel:8485655694
- Email:sales@alchempharmtech.com
- Country:United States
- ProdList:63687
- Advantage:58
-
Supplier:
CONIER CHEM AND PHARMA LIMITED
- Tel: +8618523575427
- Email:sales@conier.com
- Country:China
- ProdList:49732
- Advantage:58
-
Supplier:
Guangzhou Yuheng Pharmaceutical Technology Co., Ltd
- Tel: +8613580539051
- Email:joe@yuhengpharm.com
- Country:CHINA
- ProdList:21142
- Advantage:58
-
Supplier:
Hefei TNJ Chemical Industry Co.,Ltd.
- Tel:+86-0551-65418671<br/>+8618949823763
- Email:sales@tnjchem.com
- Country:China
- ProdList:34563
- Advantage:58
-
Supplier:
ANHUI WITOP BIOTECH CO., LTD
- Tel: +8615255079626
- Email:eric@witopchemical.com
- Country:China
- ProdList:23541
- Advantage:58
-
Supplier:
Hangzhou MolCore BioPharmatech Co.,Ltd.
- Tel:+86-057181025280;<br/>+8617767106207
- Email:sales@molcore.com
- Country:China
- ProdList:49734
- Advantage:58
-
Supplier:
Hebei Chuanghai Biotechnology Co., Ltd
- Tel: +8615531151365
- Email:mina@chuanghaibio.com
- Country:China
- ProdList:18137
- Advantage:58
-
Supplier:
LEAPCHEM CO., LTD.
- Tel:+86-852-30606658
- Email:market18@leapchem.com
- Country:China
- ProdList:43340
- Advantage:58
-
Supplier:
Sichuan Biosynce Pharmatech Co., Ltd.
- Tel: +8619950309693
- Email:diane@biosynce.com
- Country:China
- ProdList:4871
- Advantage:58
33167-21-4, Ethyl (3-chlorobenzoyl)acetateRelated Search:
- Ethyl (3-chlorobenzoyl)acetate 3-(4-CHLORO-PHENYL)-3-OXO-PROPIONIC ACID ETHYL ESTER,ETHYL 3-(4-CHLOROPHENYL)-3-OXOPROPANOATE ethyl 3-(2,5-dichlorophenyl)-3-oxopropanoate 2,4,5-TRICHLORO-BETA-OXO-BENZENEPROPANOIC ACID ETHYL ESTER 7,8-DICHLORO-4-HYDROXY-QUINOLINE-3-CARBOXYLIC ACID ETHYL ESTER 3-(3,4-DICHLORO-PHENYL)-3-OXO-PROPIONIC ACID ETHYL ESTER Ethyl 3-(3,5-dichlorophenyl)-3-oxopropanoate ETHYL 3-OXO-3-(2,3,4-TRICHLOROPHENYL)PROPANOATE ETHYL (3-CHLORO-5-FLUOROBENZOYL)ACETATE 3-(2-CHLORO-PHENYL)-3-OXO-PROPIONIC ACID ETHYL ESTER AKOS MSC-0295 AKOS 90068 RARECHEM AL BI 0740 SALOR-INT L136042-1EA RARECHEM AL BI 0440 RARECHEM AL BI 0586 RARECHEM AL BI 0716 3-(3-CHLORO-PHENYL)-2-CYANO-3-OXO-PROPIONIC ACID ETHYL ESTER
- Organic Building Blocks
- Chemical Synthesis
- Carbonyl Compounds
- C10 to C11
- Building Blocks
- Esters
- Carbonyl Compounds
- C10 to C11
- 高端化学
- 苯类
- Carbonyl Compounds
- C10 to C11
- Building Blocks
- Organic Building Blocks
- Esters
- 3-(3-氯苯基)-3-氧代-丙酸乙酯
- 3-(3-氯苯基)-3-氧代丙酸乙酯
- 3-CHLORO-脽-OXOBENZENEPROPANOIC ACID ETHYL ESTER
- (3-氯苯甲酰基)乙酸乙酯
- (3-氯苯甲酰)乙酸乙酯
- 33167-21-4
- Ethyl 3-chloro-β-oxobenzenepropanoate
- Ethyl (3-<WBR>chlorobenzoyl)<WBR>acetate
- 2-[(3-chlorophenyl)-oxomethyl]butanoate
- Benzenepropanoic acid, 3-chloro-β-oxo-, ethyl ester
- 3-(3-chlorophenyl)-3-oxopropanoic acid ethyl ester
- 3-(3-chlorophenyl)-3-keto-propionic acid ethyl ester
- Ethyl (3-chlorobenzoyl)acetate >=97%
- 3-Chloro--oxobenzenepropanoic acid ethyl ester
- Ethyl 2-(3-chlorobenzoyl)acetate
- 3-(3-CHLORO-PHENYL)-3-OXO-PROPIONIC ACID ETHYL ESTER
- ETHYL (3-CHLOROBENZOYL)ACETATE
- ETHYL 3-(3-CHLOROPHENYL)-3-OXOPROPANOATE