ChemicalBook > CAS DataBase List > ACETOHEXAMIDE
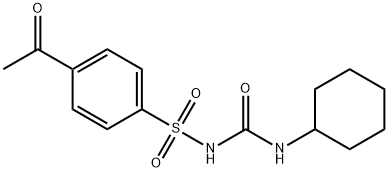
ACETOHEXAMIDE
ACETOHEXAMIDE
- CAS No.968-81-0
- Chemical Name:ACETOHEXAMIDE
- CBNumber:CB1316029
- Molecular Formula:C15H20N2O4S
- Formula Weight:324.4
- MOL File:968-81-0.mol
ACETOHEXAMIDE Property
- Melting point 188-190° (GB 912789); mp 175-177° (Marshall)
- Density 1.2528 (rough estimate)
- refractive index 1.6930 (estimate)
- storage temp. Refrigerator
- solubility DMSO: ~45 mg/mL
- form solid
- pka 4.32±0.10(Predicted)
- color white
- Water Solubility 0.25g/L(25 ºC)
- CAS DataBase Reference 968-81-0
- FDA UNII QGC8W08I6I
- NCI Drug Dictionary acetohexamide
- ATC code A10BB31
- EPA Substance Registry System Acetohexamide (968-81-0)
- UNSPSC Code 41116107
Safety
- Safety Statements :36
- WGK Germany :2
- RTECS :YR7350000
- Hazardous Substances Data :968-81-0(Hazardous Substances Data)
- Toxicity :LD50 oral in rat: > 2gm/kg
- Symbol(GHS)
- Signal word
- Hazard statements
- Precautionary statements
ACETOHEXAMIDE Price
More Price(1)
- Brand: Sigma-Aldrich(India)
- Product number: A178
- Product name : Acetohexamide
- Purity: analytical standard
- Packaging: 1G
- Price: ₹9006.4
- Updated: 2022/06/14
- Buy: Buy
ACETOHEXAMIDE Chemical Properties,Usage,Production
- Originator Dymelor ,Lilly ,US ,1964
- Uses antifungal
- Uses Labelled Acetohexamide, a sulfonylurea derivative. Acetohexamide is a hyopglycemic agent with moderate uricosuric activity. Acetohexamide is a first generation medication used in the treatment of diab etes metilus type 2.
- Uses Acetohexamide is a sulfonylurea derivative. Acetohexamide is a hyopglycemic agent with moderate uricosuric activity. Acetohexamide is a first generation medication used in the treatment of diabetes metilus type 2.
- Definition ChEBI: An N-sulfonylurea that is urea in which a hydrogen attached to one of the nitrogens is replaced by a p-acetylphenylsulfonyl group, while a hydrogen attached to the other nitrogen is replaced by a cyclohexyl group.
-
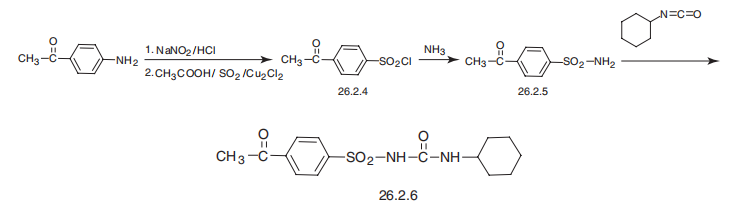
Manufacturing Process
Preparation of p-Acetylbenzenesulfonamide: 100 grams of paminoacetophenone
were dissolved in a solvent mixture containing 165 ml of
12 N hydrochloric acid and 165 ml of glacial acetic acid. The mixture was
cooled with stirring to about 0°C. A solution containing 56.2 grams of sodium
nitrite and 175 ml of water was added dropwise with stirring to the acidic
solution while maintaining the temperature below 5°C.
After the addition had been completed, the acidic solution containing pacetylphenyldiazonium chloride formed in the above reaction was added dropwise with stirring to a mixture of 530 ml of glacial acetic acid and 530 ml of benzene which had been previously cooled, and the cooled solution saturated with sulfur dioxide and to which had been added 34 g of cupric chloride dihydrate. After the addition had been completed, the reaction mixture was stirred at about 40°C for three hours, and was then poured into 3,000 ml of an ice-water mixture.
The benzene layer containing p-acetylbenzenesulfonyl chloride formed in the above reaction was separated, and the acidic aqueous phase was extracted twice with 250 ml portions of benzene. The benzene layers were combined, the combined extracts were filtered, and the benzene was evaporated from the resulting filtrate in vacuum.
The solid residue comprising p-acetylbenzenesulfonyl chloride was dissolved in 100 ml of dioxane, and the solution was added to 200 ml of 14% aqueous ammonium hydroxide. The resulting solution was stirred overnight at ambient room temperature. The p-acetylbenzenesulfonamide thus prepared was collected by filtration. Recrystallization of the filter cake from aqueous ethanol yielded purified p-acetylbenzenesulfonamide melting at about 176°C to 179°C.
Preparation of N-p-Acetylphenylsulfonyl-N'-Cyclohexylurea: A reaction mixture consisting of 32.7 grams of p-acetylbenzenesulfonamide and 64 grams of anhydrous potassium carbonate in 350 ml of anhydrous acetone was stirred at refluxing temperature for about 1% hours, thus forming the potassium salt of p-acetylbenzenesulfonamide. 30.9 grams of cyclohexylisocyanate were added dropwise to the reaction mixture. Refluxing and stirring were continued during the course of the addition and for an additional 16 hours.
The acetone was removed by evaporation in vacuum, and about 750 ml of water were added to dissolve the resulting residue. The solution was filtered. The potassium salt of N-p-acetylphenylsulfonyl-N'-cyclohexylurea formed in the above reaction, being water-soluble, passed into the filtrate. Acidification of the filtrate with 6 N aqueous hydrochloric acid caused the precipitation of N-p-acetylphenylsulfonyl-N'-cyclohexylurea which was collected by filtration. Recrystallization of the filter cake from 90% aqueous ethanol yielded purified N-p-acetylphenylsulfonyl-N'-cyclohexylurea melting at about 188-190°C. - brand name Dymelor (Lilly).
- Therapeutic Function Hypoglycemic
- General Description Acetohexamide is 4-acetyl-N-[(cyclohexylamino)carbonyl]benzenesulfonamide; or 1-[(p-acetylphenyl)sulfonyl]-3-cyclohexylurea; or 1-(p-acetylbenzenesulfonyl)-3-cyclohexylurea(generic). Acetohexamide incorporates the nearly optimal(for potency) cyclohexyl moiety in the “right-hand” sideof its molecular structure, but a p-acetyl substituent on the“left-side” benzene ring that decreases lipophilicity and israpidly biotransformed by reduction to an active metabolitethat is cleared relatively rapidly (see preceding discussion) independentlyof any P450s.
- General Description White fluffy crystalline powder with almost no odor.
- General Description Acetohexamide, 1-[(p-acetylphenyl)sulfonyl]-3-cyclohexylurea (Dymelor), is relatedchemically and pharmacologically to tolbutamide and chlorpropamide.Like the other sulfonylureas, acetohexamidelowers the blood sugar level, primarily by stimulating the releaseof endogenous insulin.
- Air & Water Reactions Water insoluble.
- Reactivity Profile An amide. Organic amides/imides react with azo and diazo compounds to generate toxic gases. Flammable gases are formed by the reaction of organic amides/imides with strong reducing agents. Amides are very weak bases (weaker than water). Imides are less basic yet and in fact react with strong bases to form salts. That is, they can react as acids. Mixing amides with dehydrating agents such as P2O5 or SOCl2 generates the corresponding nitrile. The combustion of these compounds generates mixed oxides of nitrogen (NOx).
- Fire Hazard Flash point data for ACETOHEXAMIDE are not available; however, ACETOHEXAMIDE is probably combustible.
- Biological Activity Oral hypoglycemic agent
- Clinical Use Acetohexamide is metabolized in the liver to a reducedform, the α -hydroxyethyl derivative. This metabolite, themain one in humans, possesses hypoglycemic activity.Acetohexamide is intermediate between tolbutamide andchlorpropamide in potency and duration of effect on bloodsugar levels.
- Safety Profile Human reproductive effects by an unspecified route: stillbirth. Mildly toxic by ingestion. When heated to decomposition it emits very toxic fumes of SO, and NOx,.
-
Synthesis
Acetohexamide, 1-(p-acetyl phenylsulfonyl)-3-cyclohexylurea
(26.2.6), is made in an analogous scheme by reacting p-chlorobenzenesulfonylamide with
cyclohexylisocyanate. The necessary p-acetylbenzenesulfonylamide is made by diazotating
of p-aminoacetophenone in the presence of sulfur dioxide and copper(II) chloride,
forming the sulfonylchloride 26.2.4, which is reacted further with ammonia to give the
sulfonamide (26.2.5). Reacting this with cyclohexylisocyanate gives acetohexamide
(26.2.6).
ACETOHEXAMIDE Preparation Products And Raw materials
Raw materials
Preparation Products
Global(98)Suppliers
-
Supplier:
Hubei xin bonus chemical co. LTD
- Tel:86-13657291602
- Email:linda@hubeijusheng.com
- Country:CHINA
- ProdList:22963
- Advantage:58
-
Supplier:
career henan chemical co
- Tel:+86-0371-86658258<br/>+8613203830695
- Email:factory@coreychem.com
- Country:China
- ProdList:29808
- Advantage:58
-
Supplier:
Tianjin Xinshengjiahe Science & Technology Development Co,.Ltd
- Tel:+86-86-22-87899925<br/>+86-8618522618860
- Email:18522618860@163.com
- Country:China
- ProdList:694
- Advantage:58
-
Supplier:
TargetMol Chemicals Inc.
- Tel:+1-781-999-5354<br/>+1-00000000000
- Email:marketing@targetmol.com
- Country:United States
- ProdList:32159
- Advantage:58
-
Supplier:
Dayang Chem (Hangzhou) Co.,Ltd.
- Tel:+86-0571-88938639<br/>+8617705817739
- Email:info@dycnchem.com
- Country:China
- ProdList:52849
- Advantage:58
-
Supplier:
Labnetwork lnc.
- Tel:+86-27-50766799<br/>+8618062016861
- Email:contact@labnetwork.com
- Country:China
- ProdList:19987
- Advantage:58
-
Supplier:
Shaanxi Didu New Materials Co. Ltd
- Tel:+86-89586680<br/>+86-13289823923
- Email:1026@dideu.com
- Country:China
- ProdList:8772
- Advantage:58
-
Supplier:
TargetMol Chemicals Inc.
- Tel:
- Email:support@targetmol.com
- Country:United States
- ProdList:38630
- Advantage:58
-
Supplier:
Shanghai Acmec Biochemical Technology Co., Ltd.
- Tel:+86-18621343501;<br/>+undefined18621343501
- Email:product@acmec-e.com
- Country:China
- ProdList:33338
- Advantage:58
-
Supplier:
Aladdin Scientific
- Tel:
- Email:tp@aladdinsci.com
- Country:United States
- ProdList:57505
- Advantage:58
968-81-0, ACETOHEXAMIDERelated Search:
- Amlodipine carbutamide Urea TOLAZAMIDE Glibenclamide glycyclamide N-CYCLOHEXYLFORMAMIDE 4-Methylphenylsulfonylurea Cyclohexylurea ACETOHEXAMIDE 4-Ethylbenzenesulfonamide 4-Acetylbenzenesulphonamide ANTI-ACETOHEXAMIDE acetohexamide reductase Acetohexamide-(o-carboxymethyl) oxime-KLH, Sheep anti-, Polarisation fluoroimmunoassay N-(aminocarbonyl)benzenesulphonamide 4-METHYLSULFAMYL-ACETOPHENONE
- Sulfur & Selenium Compounds
- Pharmaceuticals
- Intermediates & Fine Chemicals
- Aromatics
- ANCOBON
- 化工中间体
- 抑制剂
- 合成有机化合物配体
- 活性氧
- 标准品
- 小分子抑制剂
- 硫化物
- Analytical Standards
- Analytical Chromatography Product Catalog
- Alphabetic
- AA to AL
- C15H20N2O4S
- 醋磺己脲,10 MM DMSO 溶液
- 甲醇中醋磺己脲
- 醋酸己酰胺
- 乙酰苯磺酰环己脲
- 4-乙酰基-N-[(环己胺基)羰基]苯磺酰胺
- 1[(对乙酰苯)磺酰]-3-环已脲
- 醋磺环已脲
- 醋磺环己脲
- 1[(对乙酰苯)磺酰]-3-环己脲
- 乙酸己脲
- 4-乙酰-N-[(环己氨基)羰基]苯磺酰胺
- 醋磺己脲
- 968-81-0
- Acetohexamide, 10 mM in DMSO
- inhibit,Inhibitor,Acetohexamide
- dymelor
- Cyclamide
- Acetohexamide (1006007)
- Acetoexamide
- ACETOHEXAMIDE USP/EP/BP
- u14812
- tsiklamid
- ordimel
- nci-c03247
- n-(p-acetylphenylsulfonyl)-n’-cyclohexylurea
- minoral
- metaglucina
- hypoglicil
- gamadiabet
- gamadiaber
- dimelor
- dimelin
- acetohexamid
- 4-acetyl-n-benzenesulfonamide
- 4-acetyl-n-[(cyclohexylamino)carbonyl]-benzenesulfonamid
- 4-acetyl-n-((cyclohexylamino)carbonyl)-benzenesulfonamid
- 1-(p-acetylbenzenesulfonyl)-3-cyclohexylurea
- 1-((p-acetylphenyl)sulfonyl)-3-cyclohexylurea
- 1-((p-acetylphenyl)sulfonyl)-3-cyclohexyl-ure
- U 14812-d6
- TsiklaMid-d6