ChemicalBook > CAS DataBase List > (R)-(-)-4,12-BIS(DIPHENYLPHOSPHINO)-[2.2]-PARACYCLOPHANE
(R)-(-)-4,12-BIS(DIPHENYLPHOSPHINO)-[2.2]-PARACYCLOPHANE
(R)-(-)-4,12-BIS(DIPHENYLPHOSPHINO)-[2.2]-PARACYCLOPHANE
- CAS No.192463-40-4
- Chemical Name:(R)-(-)-4,12-BIS(DIPHENYLPHOSPHINO)-[2.2]-PARACYCLOPHANE
- CBNumber:CB1224685
- Molecular Formula:C40H34P2
- Formula Weight:576.65
- MOL File:192463-40-4.mol
(R)-(-)-4,12-BIS(DIPHENYLPHOSPHINO)-[2.2]-PARACYCLOPHANE Property
- Melting point 222-225°C
- alpha +63.2° (c 3.27, CH2Cl2)
- storage temp. under inert gas (nitrogen or Argon) at 2-8°C
- form solid
- color white
- optical activity [α]22/D +34°, c = 1 in chloroform
- CAS DataBase Reference 192463-40-4
- UNSPSC Code 12352002
- NACRES NA.22
Safety
- Hazard Codes :Xi
- Risk Statements :36/37/38-37
- Safety Statements :26-36/37/39
- WGK Germany :3
-
Symbol(GHS)

- Signal wordWarning
- Hazard statements H302-H315-H319-H335
- Precautionary statements P261-P305+P351+P338
(R)-(-)-4,12-BIS(DIPHENYLPHOSPHINO)-[2.2]-PARACYCLOPHANE Price
More Price(2)
- Brand: Sigma-Aldrich(India)
- Product number: 682136
- Product name : (S)-(+)-4,12-Bis(diphenylphosphino)-[2.2]-paracyclophane
- Purity: 96%
- Packaging: 100MG
- Price: ₹8281.13
- Updated: 2022/06/14
- Buy: Buy
- Brand: Sigma-Aldrich(India)
- Product number: 682136
- Product name : (S)-(+)-4,12-Bis(diphenylphosphino)-[2.2]-paracyclophane
- Purity: 96%
- Packaging: 500MG
- Price: ₹33871.43
- Updated: 2022/06/14
- Buy: Buy
(R)-(-)-4,12-BIS(DIPHENYLPHOSPHINO)-[2.2]-PARACYCLOPHANE Chemical Properties,Usage,Production
-
Reaction
- Ligand for rhodium-catalyzed enantioselective hydroboration of cyclopropenes.
- Ligand for iridium-catalyzed enantioselective [2+2] cycloaddition of oxabicyclic alkenes with terminal alkynes.
- Ligand for the palladium-catalyzed enantioselective hydroxycarbonylation and alkoxycarbonylation of alkenes.
- Ligand for the palladium-catalyzed amides synthesis via C(sp3 )–H bond functionalization and CO insertion.
- Ligand for iridium-catalyzed diene hydrohydroxymethylation.
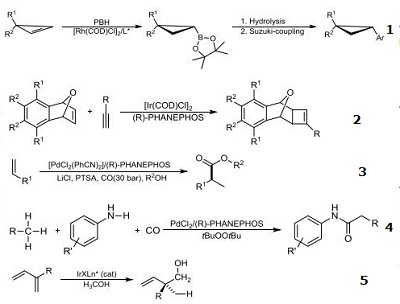
- Uses Efficient ligand for asymmetric hydrogentation of dehydroamino acids, methyl esters and keytones.
(R)-(-)-4,12-BIS(DIPHENYLPHOSPHINO)-[2.2]-PARACYCLOPHANE Preparation Products And Raw materials
Raw materials
Preparation Products
Global(44)Suppliers
-
Supplier:
Coresyn Pharmatech Co., Ltd.
- Tel:+86-571-86626709<br/>+86-18157142896
- Email:cbc@coresyn.com
- Country:China
- ProdList:9984
- Advantage:58
-
Supplier:
Aladdin Scientific
- Tel:
- Email:tp@aladdinsci.com
- Country:United States
- ProdList:57505
- Advantage:58
-
Supplier:
XIAMEN AMITY INDUSTRY AND TRADE CO., LTD.
- Tel: +8618950047208
- Email:ellena@amitychem.com
- Country:China
- ProdList:43416
- Advantage:58
- Supplier: J & K SCIENTIFIC LTD.
- Tel: 18210857532
- Email:jkinfo@jkchemical.com
- Country:China
- ProdList:96815
- Advantage:76
- Supplier: Meryer (Shanghai) Chemical Technology Co., Ltd.
- Tel:021-61259108<br/>18621169109
- Email:market03@meryer.com
- Country:China
- ProdList:40228
- Advantage:62
- Supplier: Alfa Aesar
- Tel:400-6106006
- Email:saleschina@alfa-asia.com
- Country:China
- ProdList:30123
- Advantage:84
- Supplier: Energy Chemical
- Tel:021-021-58432009<br/>400-005-6266
- Email:sales8178@energy-chemical.com
- Country:China
- ProdList:44732
- Advantage:61
- Supplier: Daicel Chiral Technologies (China)CO.,LTD
- Tel:021-50460086-9<br/>15921403865
- Email:han_yajun@dctc.daicel.com
- Country:China
- ProdList:6576
- Advantage:65
- Supplier: Bide Pharmatech Ltd.
- Tel:400-164-7117<br/>13681763483
- Email:product02@bidepharm.com
- Country:China
- ProdList:41457
- Advantage:60
- Supplier: Shanghai Macklin Biochemical Co.,Ltd.
- Tel: 15221275939
- Email:shenlinxing@macklin.cn
- Country:China
- ProdList:15874
- Advantage:55
192463-40-4, (R)-(-)-4,12-BIS(DIPHENYLPHOSPHINO)-[2.2]-PARACYCLOPHANERelated Search:
- Parylene N Diphenylphosphine 4-FLUOROBENZOYLACETONITRILE (R)-(-)-4,12-BIS(DIPHENYLPHOSPHINO)-[2.2]-PARACYCLOPHANE, MIN. 95% (R)-PHANEPHOS (R)-(-)-4,12-BIS(DIPHENYLPHOSPHINO)-[2.2]-PARACYCLOPHANE (R)-PHANEPHOS (S)-(+)-4,12-BIS(DI(3,5-XYLYL)PHOSPHINO)-[2.2]-PARACYCLOPHANE ,CTH-(S)-3,5-XYLYL-PHANEPHOS (S)-PARAPHOS RUTHENIUMCL2 (R,R)-DPEN DICHLORO[(R)-(-)-4,12-BIS(DI(3,5-XYLYL)PHOSPHINO)-[2,2]-PARACYCLOPHANE][(1S,2S)-(-)-1,2-DIPHENYLETHYLENEDIAMINE]RUTHENIUM [(R)-PARAPHOS RHODIUM (NBD)]BF4 DICHLORO[(S)-(+)-4,12-BIS(DI(3,5-XYLYL)PHOSPHINO)-[2.2]-PARACYCLOPHANE][(1R,2R)-(+)-1,2-DIPHENYLETHYLENEDIAMINE]RUTHENIUM (II) (R)-(-)-4,12-BIS(DI-3,5-XYLYLPHOSPHINO)[2.2]PARACYCLOPHANE(1,5-CYCLOOCTADIENE)RHODIUM(I) TETRAFLUOROBORATE (R)-(-)-4,12-BIS(DIPHENYLPHOSPHINO)-[2.2]-PARACYCLOPHANE
- organophosphine ligand
- PHANEPhos Series
- Chiral Phosphine
- 高端化学
- 有机配体
- 膦配体
- Chiral Catalysts, Ligands, and Reagents
- Asymmetric Synthesis
- Hydrogenation
- (S)-4,12-双(二苯基膦)-[2.2]二聚对二甲苯
- (S)-4,12-双(二苯基膦)-[2.2]二聚对二甲苯【大包装请详询客服】
- (S)-(+)-4,12-双(二苯基膦)-[2.2]-对环芳烷
- (S)-4,12-二(二苯基膦基)[2.2]对环芳烷,98%,E
- (S)-4,12-二(二苯基膦)溴[2.2]对环芳,98%,EE98%
- (S)-4,12-二(二苯基膦)溴[2.2]对环芳
- (S)-4,12-二(二苯基膦)溴[2.2]对环芳,98%,E
- (S)-4,12-二(二苯基膦)[2.2]对环芳
- (S)-4,12-双(二苯基膦)溴[2.2]对环芳烷
- (S)-4,12-二(二苯基膦基)[2.2]对环芳烷
- 192463-40-4
- (<i>S</i>)-(+)-4,12-Bis(diphenylphosphino)-[2.2]-paracyclophane
- (S)-(+)-4,12-Bis(diphenylphosphino)-[2.2]-paracyclophane, min. 95%,99%ee
- (S)-(+)-4,12-Bis(diphenylphosphino)-[2.2]-paracyclophane, min. 95%
- (S)-4,12-Bis(diphenylphosphino)-[2.2]paracyclophane,99%e.e.
- (Sp)-Phanephos
- (Sp)-(+)-4,12-Bis(diphenylphosphino)[2.2]paracyclophane
- (S)-(+)-4,12-Bis(diphenylphosphino)-[2.2]-paracyclophane, min. 97% (S)-PHANEPHOS
- (S)-(+)-4,12-Bis(D
- (S)-Phanephos, 98%, ee 98%
- (S)-(+)-4,12-BIS-(DIPHENYLPHOSPHINO)-[2.2]-PARACYCLOPHANE (S)-PHANEPHOS
- (S)-(+)-4,12-Bis(diphenylphosphino)-[2.2]-paracyclophane,min.95%(S)-PHANEPHOS
- (s)-phanephos
- 4-FLUOROBENZOYLACETONITRILE, 97% N-BOC-4-OXO-L-PROLINE, 97%
- Bisdiphenylphosphinoparacyclophane
- (R)-(-)-4,12-BIS(DIPHENYLPHOSPHINO)-[2.2]-PARACYCLOPHANE
- (R)-PHANEPHOS
- (S)-(+)-4,12-BIS(DIPHENYLPHOSPHINO)-[2.2]-PARACYCLOPHANE
![(R)-(-)-4,12-BIS(DIPHENYLPHOSPHINO)-[2.2]-PARACYCLOPHANE Structure](https://www.chemicalbook.com/CAS/GIF/192463-40-4.gif)