ChemicalBook > CAS DataBase List > Dothiepin
Dothiepin
Dothiepin
- CAS No.113-53-1
- Chemical Name:Dothiepin
- CBNumber:CB0378413
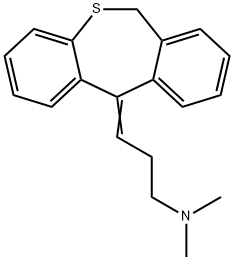
- Molecular Formula:C19H21NS
- Formula Weight:295.44
- MOL File:113-53-1.mol
Dothiepin Property
- Melting point 55-57°
- Boiling point bp0.05 171-172°
- Density 1.1022 (rough estimate)
- refractive index 1.5300 (estimate)
- storage temp. Refrigerator
- solubility Chloroform (Slightly), Methanol (Slightly)
- form Thick Oil to Low-Melting Solid
- pka 9.35±0.28(Predicted)
- color Off-White to Light Yellow
- EWG's Food Scores 1
- FDA UNII W13O82Z7HL
- ATC code N06AA16
- UNSPSC Code 41116107
- NACRES NA.24
Safety
- RIDADR :3249
- HazardClass :6.1(b)
- PackingGroup :III
- Hazardous Substances Data :113-53-1(Hazardous Substances Data)
- Toxicity :LD50 oral in rat: 450mg/kg
-
Symbol(GHS)


- Signal wordWarning
- Hazard statements H302-H361-H362
- Precautionary statements P201-P260-P263-P264-P280-P308+P313
Dothiepin Chemical Properties,Usage,Production
- Chemical Properties Pale Yellow Low Melting Solid
- History Described in the 1960s by the Czech company Sdruzeni Podniku pro Zdravotnickon Vyrobu; SPOFA (Spofa, 1962)
- Uses A tricyclic antidepressant.
- Definition ChEBI: Dothiepin is a dibenzothiepine. It has a role as an antidepressant and an anticoronaviral agent.
-
Synthesis Reference(s)
Synthesis (Spofa, 1962): S-Benzylthiosalicylic acid is treated with polyphosphoric acid and the resulting cyclic ketone is reacted with 3-dimethylaminopropyl magnesium chloride to afford an alcohol which is dehydrated with sulfuric acid.
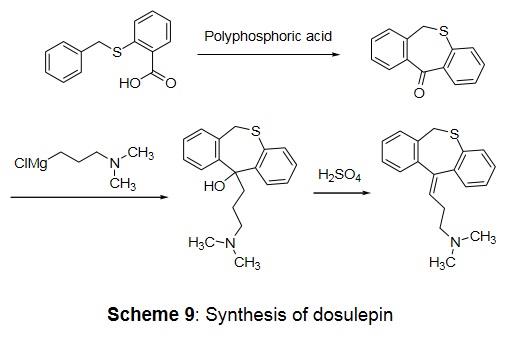
Synthesis of dosulepin - Clinical Use Dosulepin (also referred to as dothiepin) is a member of the TCA family and has similar clinical uses as amitrip- tyline, thus providing effective treatment of depression (Goldstein and Claghorn, 1980; Lancaster and Gonzales, 1989; Donovan et al., 1991) and also against pain (Feinmann et al., 1984; Caruso et al., 1987) and tinnitus. Like amitriptyline it has sedative properties, however its anti-muscarinic side-effects are less pronounced. Dosulepin is readily absorbed from the GI tract and extensively demethylated while undergoing a first-pass effect. Metabolic pathways include next to hydroxylation and N-oxidation steps, S-oxidation. Elimination half-lifes vary from 14 to 24 hours interindividually (Maguire et al., 1982; Yu et al., 1986; Ilett et al., 1993).
Dothiepin Preparation Products And Raw materials
Raw materials
Preparation Products
Global(48)Suppliers
-
Supplier:
Hubei Jusheng Technology Co.,Ltd.
- Tel:18871490254
- Email:linda@hubeijusheng.com
- Country:CHINA
- ProdList:28172
- Advantage:58
-
Supplier:
Xi'an MC Biotech, Co., Ltd.
- Tel:029-89275612<br/>+8618991951683
- Email:mcbio_sales@163.com
- Country:China
- ProdList:2251
- Advantage:58
-
Supplier:
AFINE CHEMICALS LIMITED
- Tel:+86-0571-85134551
- Email:sales@afinechem.com
- Country:China
- ProdList:15393
- Advantage:58
-
Supplier:
Shaanxi Didu New Materials Co. Ltd
- Tel:+86-89586680<br/>+86-13289823923
- Email:1026@dideu.com
- Country:China
- ProdList:8795
- Advantage:58
-
Supplier:
Hebei Fengjia New Material Co., Ltd
- Tel:+86-0311-87836622<br/>+86-18712993135
- Email:sales01@tairunfaz.com
- Country:China
- ProdList:8051
- Advantage:58
-
Supplier:
Hangzhou MolCore BioPharmatech Co.,Ltd.
- Tel:+86-057181025280;<br/>+8617767106207
- Email:sales@molcore.com
- Country:China
- ProdList:49734
- Advantage:58
-
Supplier:
GIHI CHEMICALS CO.,LIMITED
- Tel: +8618058761490
- Email:info@gihichemicals.com
- Country:China
- ProdList:49984
- Advantage:58
-
Supplier:
TargetMol Chemicals Inc.
- Tel: +8613564774135
- Email:zijue.cai@tsbiochem.com
- Country:United States
- ProdList:19888
- Advantage:58
- Supplier: J & K SCIENTIFIC LTD.
- Tel: 18210857532
- Email:jkinfo@jkchemical.com
- Country:China
- ProdList:94657
- Advantage:76
- Supplier: Chemsky(shanghai)International Co.,Ltd.
- Tel:021-50135380
- Email:shchemsky@sina.com
- Country:China
- ProdList:32321
- Advantage:50
113-53-1, DothiepinRelated Search:
- Citalopram Trazodone CIS,TRANS-DOTHIEPIN-D3,100/MLINMETHANOL dothiepin sulfoxide,Dothiepin S-oxide DOTHIEPIN HYDROCHLORIDE,DOTHIEPIN HCL,Dothiepin·hydrochloric acid,DOSULEPIN HYDROCHLORIDE (DOTHIEPIN HYDROCHLORIDE) DOSULEPIN (DOTHIEPIN) HYDROCHLORIDE DOTHIEPIN-D3 11-(1-Methyl-4-piperidylidene)-6,11-dihydrodibenzo(b,e)thiepine-9-carb oxylic acid HCl (E)-9-Fluoro-11-(3-dimethylaminopropylidene)-6,11-dihydrodibenzo(b,e)t hiepin hydrogen maleate Dothiepin 3-dibenzo[b,e]thiepin-11(6H)-ylidene-8-methyl-8-azabicyclo[3.2.1]octane hydrochloride Tropatepine
- Sulfur & Selenium Compounds
- Pharmaceuticals
- Intermediates & Fine Chemicals
- Amines
- Chemical Amines
- 抑制剂
- 医药原料
- 神经系统用药
- 度硫平
- 多硫平-D3马来酸盐
- 度琉平
- 113-53-1
- Dothiepin-d3 Maleate
- 3-(6,11-dihydrodibenzo(b,e)thiepin-11-ylidene)propyldimethylamine
- DOTHIEPIN
- N,N-Dimethyl- dibenzo[b,e]thiepin-11(6H),-propylamine
- 3-Dibenzo[b,e]thiepin-11(6H)-ylidene-N,N-dimethyl-1-propanamine
- CIS,TRANS-DOTHIEPIN,1.0MG/MLINMETHANOL
- Methiaden
- Dolsulepine
- DOTHIEPIN,3-DIBENZO[B,E] THIEPIN-11-(6H)-YLIDENE-N,N-DIMETHYL-PROPONAMINE
- 3-Dibenzo[b,e]thiepin-11(6H)-ylidene-N,N-diMethyl-
- Dothiepin (cis/trans)
- (E)-3-[Dibenzo[b,e]thiepin-11(6H)-ylidene]-N,N-dimethylpropane-1-amine
- (E)-3-[Dibenzo[b,e]thiepin-11(6H)-ylidene]-N,N-dimethyl-1-propanamine
- 3-DIBENZO[B,E] THIEPIN-11-(6H)-YLIDENE-N,N-DIMETHYL-PROPONAMINE
- Prothiadene
- Prothiaden spofa
- Prothiaden
- Propylamine, N,N-dimethyl-3-(dibenzo(b,e)thiepin-delta-sup(11(6H),gamma))-
- N,N-Dimethyldibenzo(b,e)thiepin-delta-sup(11(6H),gamma)propylamine
- n,n-dimethyl-3-(dibenzo(b,e)thiepin-delta-sup(11(6h),gamma))-propylamin
- iz914
- IZ 914
- Dothep
- Dosulepine
- dosulepin
- Dibenzo[b,e]thiepin-delta11(6H),gamma-propylamine, N,N-dimethyl-
- Dibenzo[b,e]thiepin, 1-propanamine deriv.
- 3-Dibenzo[b,e]thiepin-11(6H)-ylidine-N,N-dimethyl-propanamine
- 3-dibenzo(b,e)thiepin-11(6h)-ylidene-n,n-dimethyl-1-propanamin
- 3-Dibenzo(b,e)thiepin-11(6H)-ylidene-N,N-dimethyl-1-propamine
- 1-Propanamine, 3-dibenzo[b,e]thiepin-11(6H)-ylidene-N,N-dimethyl-
- 11-(3-Dimethylaminopropylidene)-6,11-dihydrodibenzo(b,e)thiepin
- (3E)-3-Dibenzo[b,E]thiepin-11(6H)-ylidene-N,N-dimethyl-1-propanamine