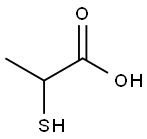
ChemicalBook > CAS DataBase List > 2-Mercaptopropionic acid
2-Mercaptopropionic acid
2-Mercaptopropionic acid
- CAS No.79-42-5
- Chemical Name:2-Mercaptopropionic acid
- CBNumber:CB0239767
- Molecular Formula:C3H6O2S
- Formula Weight:106.14
- MOL File:79-42-5.mol
2-Mercaptopropionic acid Property
- Melting point 10-14 °C (lit.)
- Boiling point 102 °C/16 mmHg (lit.) 203-208 °C (lit.)
- Density 1.196 g/mL at 25 °C (lit.)
- vapor pressure 1.7 hPa (20 °C)
- FEMA 3180 | 2-MERCAPTOPROPIONIC ACID
- refractive index n
20/D 1.481(lit.) - Flash point 190 °F
- storage temp. 2-8°C
- solubility 1g/L in organic solvents at 20 ℃
- form Liquid
- pka pK1:4.32(0);pK2:10.20(SH) (25°C)
- color Clear colorless to slightly yellow
- PH 2 (H2O, 20℃)(undiluted)
- Odor at 0.01 % in propylene glycol. meaty sulfurous roasted
- Odor Type sulfurous
- biological source synthetic
- Water Solubility MISCIBLE
- JECFA Number 551
- Merck 14,9340
- BRN 506218
- InChIKey PMNLUUOXGOOLSP-UHFFFAOYSA-N
- LogP 1.15 at 20℃
- Substances Added to Food (formerly EAFUS) 2-MERCAPTOPROPIONIC ACID
- CAS DataBase Reference 79-42-5(CAS DataBase Reference)
- EWG's Food Scores 1
- FDA UNII O5U6967KGF
- NIST Chemistry Reference 2-Mercaptopropanoic acid(79-42-5)
- EPA Substance Registry System Propanoic acid, 2-mercapto- (79-42-5)
- UNSPSC Code 12352106
- NACRES NA.22
Safety
- Hazard Codes :C
- Risk Statements :34-22
- Safety Statements :26-36/37/39-45-25
- RIDADR :UN 2936 6.1/PG 2
- WGK Germany :1
- RTECS :UF5250000
- TSCA :Yes
- HazardClass :6.1
- PackingGroup :II
- HS Code :29309070
- Toxicity :LD50 orally in Rabbit: 730 mg/kg
-
NFPA 704:
2 3 0
-
Symbol(GHS)

- Signal wordDanger
- Hazard statements H314
- Precautionary statements P280-P303+P361+P353-P304+P340+P310-P305+P351+P338-P363-P405
2-Mercaptopropionic acid Price
More Price(13)
- Brand: Sigma-Aldrich(India)
- Product number: W318000
- Product name : 2-Mercaptopropionic acid
- Purity: ≥95%, FG
- Packaging: 1SAMPLE-K
- Price: ₹5141.88
- Updated: 2022/06/14
- Buy: Buy
- Brand: Sigma-Aldrich(India)
- Product number: T31003
- Product name : Thiolactic acid
- Purity: 95%
- Packaging: 5G
- Price: ₹2035.1
- Updated: 2022/06/14
- Buy: Buy
- Brand: Sigma-Aldrich(India)
- Product number: W318000
- Product name : 2-Mercaptopropionic acid
- Purity: ≥95%, FG
- Packaging: 1KG
- Price: ₹13076.6
- Updated: 2022/06/14
- Buy: Buy
- Brand: Sigma-Aldrich(India)
- Product number: T31003
- Product name : Thiolactic acid
- Purity: 95%
- Packaging: 100G
- Price: ₹2565.53
- Updated: 2022/06/14
- Buy: Buy
- Brand: Sigma-Aldrich(India)
- Product number: T31003
- Product name : Thiolactic acid
- Purity: 95%
- Packaging: 500G
- Price: ₹8454.33
- Updated: 2022/06/14
- Buy: Buy
2-Mercaptopropionic acid Chemical Properties,Usage,Production
- Uses 2-Mercaptopropionic acid is an important raw material for organic synthesis, mainly used in the preparation of herbicides, plasticized heat stabilizers and antioxidants, as well as the deployment of daily spices.
- Preparation It is prepared from the electrolysis of the corresponding sulfide S (SCHMeCO2H) Alternatively, it can be prepared from the reaction between pyruvate and hydrogen sulfide. Hydrogen sulfide is added to 50% pyruvic acid to make it saturated at 60-70 DEG C; add hydrochloric acid to until turbidity is formed; dilute sulfuric acid is added to make it weakly acidic, and 2.5% sodium amalgam is added to the precipitated compound for reduction. Cool it till hydrogen sulfide is completed released. Then add ether the acidic medium, extract the lactic acid, remove the ether by distillation, here thiolactic acid is finally obtained.
- Content Assay Accurately weigh 1 g of sample, transfer it into a 250ml Erlenmeyer flask filled with 75 ml of water; add phenolphthalein indicator (TS-167); use 0.5mol / L NaOH solution for titration. Each ml of 0.5mol / L NaOH solution is equivalent to 53.08 mg of 2-mercaptopropionic acid.
- Storage and transportation Store in a cool and ventilated place, the container should be sealed to prevent damp, heat, handling gently, can’t be inverted, and be careful not to damage the packaging.
- Chemical Properties Thiolactic acid has a roasted, meaty odor.
- Chemical Properties Clear colorless to slightly yellow liquid. Oily liquid; Melting point 10 ℃; Boiling point d-95 ~ 100 ℃ / 2133Pa, dl- 98.5 ~ 99.0 ℃ / 1866Pa; Density I-D19.24 1.193; Refractive index dl-nD: 1.4823; Optical rotation[α] D-45.47; soluble in water, ethanol and ether.
- Uses Depilatory, hair-waving preparations.
-
Uses
Thiolactic acid (TLA) can be used as a building block in the synthesis of:
- Thiolactomycin via oxathiolanone intermediate.
- 4-Thiazolidinones by reacting various Schiff bases with thioglycolic acid.
- 1,4-Naphthoquinone derivatives containing sulfur atom for antibacterial and antiviral activity studies.
It can also be used as a bidental chelating agent for the surface modification of titanium dioxide (TiO2) nanoparticles for the removal of cadmium from waste water. - Definition ChEBI: 2-mercaptopropanoic acid is a mercaptopropanoic acid.
- Preparation By electrolysis of the corresponding sulfide, S(SCHMeCO2H)2.
- Taste threshold values Taste characteristics at 15 ppm: meaty, sulfury, brothy, and brown.
- General Description An oily liquid with an unpleasant odor. Toxic by ingestion and skin absorption. Used as a depilatory and in hair waving preparations.
- Air & Water Reactions Soluble in water and denser than water.
- Reactivity Profile 2-Mercaptopropionic acid is an organosulfide/organic acid. Organosulfides are incompatible with acids, diazo and azo compounds, halocarbons, isocyanates, aldehydes, alkali metals, nitrides, hydrides, and other strong reducing agents. Reactions with these materials generate heat and in many cases hydrogen gas. Many of these compounds may liberate hydrogen sulfide upon decomposition or reaction with an acid. Carboxylic acids donate hydrogen ions if a base is present to accept them. They react in this way with all bases, both organic (for example, the amines) and inorganic. Their reactions with bases, called "neutralizations", are accompanied by the evolution of substantial amounts of heat. Neutralization between an acid and a base produces water plus a salt. Carboxylic acids with six or fewer carbon atoms are freely or moderately soluble in water; those with more than six carbons are slightly soluble in water. Soluble carboxylic acid dissociate to an extent in water to yield hydrogen ions. The pH of solutions of carboxylic acids is therefore less than 7.0. Many insoluble carboxylic acids react rapidly with aqueous solutions containing a chemical base and dissolve as the neutralization generates a soluble salt. Carboxylic acids in aqueous solution and liquid or molten carboxylic acids can react with active metals to form gaseous hydrogen and a metal salt. Such reactions occur in principle for solid carboxylic acids as well, but are slow if the solid acid remains dry. Even "insoluble" carboxylic acids may absorb enough water from the air and dissolve sufficiently in 2-Mercaptopropionic acid to corrode or dissolve iron, steel, and aluminum parts and containers. Carboxylic acids, like other acids, react with cyanide salts to generate gaseous hydrogen cyanide. The reaction is slower for dry, solid carboxylic acids. Insoluble carboxylic acids react with solutions of cyanides to cause the release of gaseous hydrogen cyanide. Flammable and/or toxic gases and heat are generated by the reaction of carboxylic acids with diazo compounds, dithiocarbamates, isocyanates, mercaptans, nitrides, and sulfides. Carboxylic acids, especially in aqueous solution, also react with sulfites, nitrites, thiosulfates (to give H2S and SO3), dithionites (SO2), to generate flammable and/or toxic gases and heat. Their reaction with carbonates and bicarbonates generates a harmless gas (carbon dioxide) but still heat. Like other organic compounds, carboxylic acids can be oxidized by strong oxidizing agents and reduced by strong reducing agents. These reactions generate heat. A wide variety of products is possible. Like other acids, carboxylic acids may initiate polymerization reactions; like other acids, they often catalyze (increase the rate of) chemical reactions.
- Health Hazard TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
- Fire Hazard Combustible material: may burn but does not ignite readily. When heated, vapors may form explosive mixtures with air: indoors, outdoors and sewers explosion hazards. Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated. Runoff may pollute waterways. Substance may be transported in a molten form.
- Safety Profile Poison by ingestion. Moderately toxic by inhalation. When heated to decomposition it emits toxic fumes of SOx. See also SULFIDES and MERCAPTANS.
2-Mercaptopropionic acid Preparation Products And Raw materials
Raw materials
Preparation Products
Global(258)Suppliers
-
Supplier:
Hebei Chuanghai Biotechnology Co., Ltd
- Tel: +8615531157085
- Email:abby@chuanghaibio.com
- Country:China
- ProdList:8808
- Advantage:58
-
Supplier:
Hebei Mujin Biotechnology Co.,Ltd
- Tel:+86 13288715578<br/>+8613288715578
- Email:sales@hbmojin.com
- Country:China
- ProdList:12809
- Advantage:58
-
Supplier:
Hebei Chuanghai Biotechnology Co,.LTD
- Tel: +86-13131129325
- Email:sales1@chuanghaibio.com
- Country:China
- ProdList:5868
- Advantage:58
-
Supplier:
Henan Tianfu Chemical Co.,Ltd.
- Tel:+86-0371-55170693<br/>+86-19937530512
- Email:info@tianfuchem.com
- Country:China
- ProdList:21628
- Advantage:55
-
Supplier:
career henan chemical co
- Tel:+86-0371-86658258<br/>+8613203830695
- Email:sales@coreychem.com
- Country:China
- ProdList:29859
- Advantage:58
-
Supplier:
Hubei Jusheng Technology Co.,Ltd.
- Tel:18871490254
- Email:linda@hubeijusheng.com
- Country:CHINA
- ProdList:28172
- Advantage:58
-
Supplier:
Xiamen AmoyChem Co., Ltd
- Tel:+86-86-5926051114<br/>+8615060885618
- Email:sales@amoychem.com
- Country:China
- ProdList:6383
- Advantage:58
-
Supplier:
Henan Xiangtong Chemical Co., Ltd.
- Tel:86-371-61312303
- Email:info@hnxtchem.com
- Country:CHINA
- ProdList:292
- Advantage:58
-
Supplier:
Hubei xin bonus chemical co. LTD
- Tel:86-13657291602
- Email:linda@hubeijusheng.com
- Country:CHINA
- ProdList:22963
- Advantage:58
-
Supplier:
CONIER CHEM AND PHARMA LIMITED
- Tel: +8618523575427
- Email:sales@conier.com
- Country:China
- ProdList:49732
- Advantage:58
79-42-5, 2-Mercaptopropionic acidRelated Search:
- 2-Thiobarbituric acid Thioacetamide Sodium thiosulfate pentahydrate 2-Pyridinethione Sodium thiosulfate Thiophenethiol 2-Ethylhexyl mercaptoacetate Ethyl 2-(Chlorosulfonyl)acetate 3-MERCAPTOPROPIONIC ACID,BETA-MERCAPTOPROPIONIC ACID,β-Mercaptopropionic acid,3-MERCAPTOPROPIONIC ACID, 99+% Calcium thioglycolate Mercaptoacetic acid 6-Mercaptopurine Ammonium thiosulfate Citric acid Glycine Folic acid Sodium thioglycolate Thioacetic acid
- Thiols/Mercaptans
- Sulfur Compounds
- Organic Building Blocks
- Chemical Synthesis
- Building Blocks
- acid Flavor
- 高端化学
- 有机类
- 化工产品-有机化工
- 杂质对照品
- 香料原料
- 天然等同香料和人造香
- 食用香料(增香剂)
- 酸类香料
- 有机产品
- 精细化工品
- 有机原料
- 有机酸
- 化工材料
- 新材料
- 医药、农药及染料中间体
- 材料中间体及助剂
- 其他
- 原料
- 有机化工原料
- 化工
- 化工原料
- 酸类
- 含硫类
- 食品添加剂
- 巯基化合物
- 硫醇类
- 医药原料
- 硫化合物
- Building Blocks
- Sulfur Compounds
- Thiols/Mercaptans
- Organic Building Blocks
- C3H3O2S
- CH3CHSHCOOH
- CH3CSHCOOH
- 2-巯基丙酸(硫代乳酸)标准品
- 硫普罗宁杂质4
- 硫普罗宁杂质Ⅱ
- 硫普罗宁杂质II
- 2-巯基丙酸(硫普罗宁杂质)
- 硫普罗宁杂质B
- 2-巯基丙酸,97%
- 2-巯基丙酸(硫代乳酸)
- 硫代乳酸79-42-5/2-硫代乳酸
- 硫代乳酸,2-巯基丙酸
- 硫代乳酸
- 硫羟乳酸
- 2-巯基丙酸/2-硫代乳酸
- Α-巰丙酸
- 巯基乳酸
- 2-巯基丙酸
- 2-硫羟丙酸