ChemicalBook > CAS DataBase List > Methyl thioglycolate
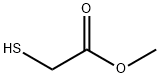
Methyl thioglycolate
Methyl thioglycolate
- CAS No.2365-48-2
- Chemical Name:Methyl thioglycolate
- CBNumber:CB0165491
- Molecular Formula:C3H6O2S
- Formula Weight:106.14
- MOL File:2365-48-2.mol
Methyl thioglycolate Property
- Melting point -24 °C
- Boiling point 42-43 °C/10 mmHg (lit.)
- Density 1.187 g/mL at 25 °C (lit.)
- refractive index n
20/D 1.466(lit.) - Flash point 86 °F
- storage temp. Store below +30°C.
- solubility 40g/l
- form Liquid
- pka 8.04±0.10(Predicted)
- color Clear colorless
- Odor Strong, unpleasant, garlic-like
- Water Solubility 40 g/L (20 ºC)
- Sensitive Air Sensitive
- BRN 506259
- InChIKey MKIJJIMOAABWGF-UHFFFAOYSA-N
- CAS DataBase Reference 2365-48-2(CAS DataBase Reference)
- EWG's Food Scores 1-3
- FDA UNII O1608LA9EL
- NIST Chemistry Reference Acetic acid, mercapto-, methyl ester(2365-48-2)
- EPA Substance Registry System Acetic acid, mercapto-, methyl ester (2365-48-2)
- UNSPSC Code 12352100
- NACRES NA.22
Safety
- Hazard Codes :Xn,T
- Risk Statements :10-20/22-36/37/38-25
- Safety Statements :26-36/37-45-36/37/39-28A
- RIDADR :UN 1992 3/PG 3
- WGK Germany :1
- RTECS :AI7350000
- TSCA :Yes
- HazardClass :3
- PackingGroup :III
- HS Code :29309070
- Hazardous Substances Data :2365-48-2(Hazardous Substances Data)
- Toxicity :mouse,LD50,intraperitoneal,100mg/kg (100mg/kg),National Technical Information Service. Vol. AD277-689,
-
NFPA 704:
2 2 0
-
Symbol(GHS)


- Signal wordDanger
- Hazard statements H226-H301-H315-H319-H332-H335
- Precautionary statements P210-P233-P301+P310-P303+P361+P353-P304+P340+P312-P305+P351+P338
Methyl thioglycolate Price
More Price(7)
- Brand: Sigma-Aldrich(India)
- Product number: 108995
- Product name : Methyl thioglycolate
- Purity: 95%
- Packaging: 5G
- Price: ₹2294.9
- Updated: 2022/06/14
- Buy: Buy
- Brand: Sigma-Aldrich(India)
- Product number: 108995
- Product name : Methyl thioglycolate
- Purity: 95%
- Packaging: 100G
- Price: ₹2413.98
- Updated: 2022/06/14
- Buy: Buy
- Brand: Sigma-Aldrich(India)
- Product number: 108995
- Product name : Methyl thioglycolate
- Purity: 95%
- Packaging: 500G
- Price: ₹5066.1
- Updated: 2022/06/14
- Buy: Buy
- Brand: TCI Chemicals (India)
- Product number: T1287
- Product name : Methyl Thioglycolate
- Purity: min. 98.0 %
- Packaging: 25G
- Price: ₹1500
- Updated: 2022/05/26
- Buy: Buy
- Brand: TCI Chemicals (India)
- Product number: T1287
- Product name : Methyl Thioglycolate
- Purity: min. 98.0 %
- Packaging: 100G
- Price: ₹1900
- Updated: 2022/05/26
- Buy: Buy
Methyl thioglycolate Chemical Properties,Usage,Production
- Chemical Properties CLEAR COLOURLESS LIQUID
- Uses Methyl Thioglycolate is an derivative of Thioglycolic Acid (T350760), an organic compound containing both a thiol and a carboxylic acid functional groups. Thioglycolic Acid and its derivatives are oft en used in organic synthesis as a nucleophile in thioglycolysis reactions and is also used as a S transfer agent for sulfonyl chloride synthesis. Methyl Thioglycolate have been studied for the its inf luence on neocarzinostatin activation and expression of DNA damage.
- Uses Methyl thioglycolate was used in the preparation of 3-carbomethoxy-4- oxotetrahydrothiopyran, 2- and 4-carbomethoxy-3-oxotetrahydrothiophene. It is used to prepare methyl thioglycolate and aminoethanethiol conjugated gold nanorods.
- Production Methods Adding thioglycollic acid with purity of between 70.0 and 96.0 percent and methanol with purity of between 95.0 and 99.9 percent into a reaction device according to the molar ratio of 1:1.3-4.0; subsequently, adding an activating agent into the reaction device, stirring and heating for reaction; and after the reaction is finished, carrying out separatory distillation on reaction products to obtain the methyl thioglycolate. The activating agent is a p-toluenesulfonic acid methanol solution with a mass concentration of between 20 and 30 percent, and the use amount is 0.5 to 1.0 percent of the total mass of relative input materials.
- Application Methyl thioglycolate is a chemical compound that is used in the pharmaceutical industry as an intermediate. It has been used to synthesize anti-inflammatory drugs, such as aspirin and ibuprofen, and also for the treatment of autoimmune diseases. Methyl thioglycolate binds to amino acids on the surface of bacteria, which prevents them from functioning properly. This binding causes the cells to stop growing and die.
- Definition Methyl thioglycolate are widely used in free-radical photoinitiated polymerization reactions. In particular, the use of methyl thioglycolate (MTG), CH3OC(O)CH2SH, results in greater photopolymerization reaction rates, attributed to a weakening of the sulfur–hydrogen bond by hydrogen bonding of the thiol group. MTG constitutes the smaller exponent of the series of CH3OC(O)(CH2)nSH compounds, reported as intermediated in the organic sulfur cycle in marine environments, produced by marine phytoplankton.
- General Description Methyl thioglycolate reacts with nonprotein component of the antitumor antibiotic neocarzinostatin to form 1:1 adduct. It reacts with isothiocyanate to form Rhodanine.
-
reaction suitability
reagent type: ligand
reaction type: [1,2]-Wittig Rearrangement
Methyl thioglycolate Preparation Products And Raw materials
Raw materials
Preparation Products
Global(422)Suppliers
-
Supplier:
Qingdao Zhongke Huatai Industrail Co., Ltd.
- Tel:+86-532-86898076<br/>+86-15153292017
- Email:zkhtchem@163.com
- Country:China
- ProdList:25
- Advantage:58
-
Supplier:
Jinan Qinmu Fine Chemical Co.,Ltd.
- Tel: +8618660799346
- Email:sales@qinmuchem.com
- Country:China
- ProdList:1009
- Advantage:58
-
Supplier:
Qingdao RENAS Polymer Material Co., Ltd.
- Tel:+86-0532-86867058<br/>+86-18562606086
- Email:sales@qdrenas.com
- Country:China
- ProdList:778
- Advantage:58
-
Supplier:
Hebei Chuanghai Biotechnology Co., Ltd
- Tel:+86-15531157085<br/>+86-15531157085
- Email:abby@chuanghaibio.com
- Country:China
- ProdList:8808
- Advantage:58
-
Supplier:
Hebei Yanxi Chemical Co., Ltd.
- Tel: +8617531153977
- Email:allison@yan-xi.com
- Country:China
- ProdList:5854
- Advantage:58
-
Supplier:
Hebei Chuanghai Biotechnology Co,.LTD
- Tel: +86-13131129325
- Email:sales1@chuanghaibio.com
- Country:China
- ProdList:5870
- Advantage:58
-
Supplier:
Hebei Fengjia New Material Co., Ltd
- Tel:+86-0311-87836622<br/>+86-18712993135
- Email:sales01@tairunfaz.com
- Country:China
- ProdList:8051
- Advantage:58
-
Supplier:
Hebei Chuanghai Biotechnology Co., Ltd
- Tel: +8615531151365
- Email:mina@chuanghaibio.com
- Country:China
- ProdList:18137
- Advantage:58
-
Supplier:
Henan Bao Enluo International TradeCo.,LTD
- Tel:+86-17331933971<br/>+86-17331933971
- Email:deasea125996@gmail.com
- Country:China
- ProdList:2472
- Advantage:58
-
Supplier:
Henan Fengda Chemical Co., Ltd
- Tel:+86-371-86557731<br/>+86-13613820652
- Email:info@fdachem.com
- Country:China
- ProdList:20237
- Advantage:58
2365-48-2, Methyl thioglycolateRelated Search:
- Kresoxim-methyl POTASSIUM THIOGLYCOLATE Methyl 3-mercaptopropionate Methyl acrylate Methyl acetate Methyl glycolate 2-Ethylhexyl mercaptoacetate Ammonium thioglycolate ETHYL THIOGLYCOLATE Methylparaben METSULFURON METHYL Bensulfuron methyl Calcium thioglycolate Mercaptoacetic acid CYANOACETIC ACID-OSU calcium sulphidoacetate Thiophanate-methyl Methyl Methyl Mercaptoacetate
- 2365-48-2
- 黄金
- 有机化工
- 杂质对照品
- 其它原料及中间体
- 高端化学
- 有机类
- 原料药
- 有机产品
- 化学试剂-有机试剂
- 液体
- 化工产品
- 化学试剂
- 化工材料
- 有机羧酸及其衍生物
- 有机中间体
- 其他原料
- 化工原料
- 化工
- 巯基乙酸甲酯
- 有机化工原料
- 有机化工原料
- 酯
- 通用试剂
- 酯类
- 有机原料
- 羧酸衍生物
- 氨基酸及其衍生物
- 有机砌块
- 羰基化合物
- 农药中间体
- 磺酰脲类除草剂
- 除草剂中间体
- Building Blocks
- Sulfur Compounds
- Thiols/Mercaptans
- Organic Building Blocks
- HSCH2COOCH3
- HSCH2CO2CH3
- 阿替卡因杂质3
- 阿替卡因标准品003
- 硫代乙醇酸甲脂
- 2-巯基乙酸甲酯
- 巯基乙酸甲酯,98%
- 巯基乙酸甲酯,95%
- 巯基乙酸甲酯 1KG
- 硫代乙醇酸甲酯
- 巯基乙酸甲酯
- 2365-48-2
- Tetramethylammonium hydroxide pentahydrate 10424-65-4
- MTG
- Methyl 2-mercaptoacetate
- THIOGLYCOLIC ACID METHYL ESTER
- METHYL MERCAPTOACETATE
- MERCAPTOACETIC ACID METHYL ESTER
- METHYL THIOGLYCOLATE Extra Pure
- Articaine-003
- Methyl thioglycolate Joyce