ChemicalBook > CAS DataBase List > HATU
HATU
HATU
- CAS No.148893-10-1
- Chemical Name:HATU
- CBNumber:CB0122630
- Molecular Formula:C10H15F6N6OP
- Formula Weight:380.24
- MOL File:148893-10-1.mol
HATU Property
- Melting point 183-188 °C (dec.)
- RTECS XZ5633000
- storage temp. 2-8°C
- solubility >16mg/mL in DMSO
- form powder to crystaline
- color White to Almost white
- Water Solubility Soluble in acetonitrile. Insoluble in water.
- InChI InChI=1S/C10H15N6O.F6P/c1-13(2)10(14(3)4)15-8-6-5-7-11-9(8)16(17)12-15;1-7(2,3,4,5)6/h5-7H,1-4H3;/q+1;-1
- InChIKey KZAWCZZRROLLDL-UHFFFAOYSA-N
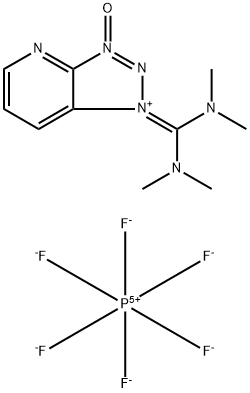
- SMILES [P+5]([F-])([F-])([F-])([F-])([F-])[F-].C(=[N+]1/N=N(=O)C2=NC=CC=C/12)(\N(C)C)/N(C)C
- CAS DataBase Reference 148893-10-1(CAS DataBase Reference)
- FDA UNII B93RIH1T7E
- EPA Substance Registry System 1H-1,2,3-Triazolo[4,5-b]pyridinium, 1-[bis(dimethylamino)methylene]-, hexafluorophosphate(1-), 3-oxide (148893-10-1)
- UNSPSC Code 12352302
- NACRES NA.22
Safety
- Hazard Codes :Xi,Xn,E
- Risk Statements :36/37/38-20/21/22-2
- Safety Statements :26-37/39-36/37-36-35
- RIDADR :1325
- WGK Germany :3
- F :10-21
- HazardClass :4.1
- PackingGroup :Ⅱ
- HS Code :29339999
-
NFPA 704:
2 2 0
-
Symbol(GHS)

- Signal wordDanger
- Hazard statements H317-H334
- Precautionary statements P261-P272-P280-P284-P302+P352-P304+P340+P312
HATU Price
More Price(23)
- Brand: Sigma-Aldrich(India)
- Product number: 8.51013
- Product name : HATU
- Purity: Novabiochem?
- Packaging: 25G
- Price: ₹37050
- Updated: 2022/06/14
- Buy: Buy
- Brand: Sigma-Aldrich(India)
- Product number: 8.51013
- Product name : HATU
- Purity: Novabiochem?
- Packaging: 100G
- Price: ₹59350
- Updated: 2022/06/14
- Buy: Buy
- Brand: Sigma-Aldrich(India)
- Product number: 8.51013
- Product name : HATU
- Purity: Novabiochem?
- Packaging: 8510130500
- Price: ₹87710
- Updated: 2022/06/14
- Buy: Buy
- Brand: Sigma-Aldrich(India)
- Product number: 8.51013
- Product name : HATU
- Purity: Novabiochem?
- Packaging: 8510131000
- Price: ₹155850
- Updated: 2022/06/14
- Buy: Buy
- Brand: Sigma-Aldrich(India)
- Product number: 445460
- Product name : HATU
- Purity: 97%
- Packaging: 1G
- Price: ₹8887.33
- Updated: 2022/06/14
- Buy: Buy
HATU Chemical Properties,Usage,Production
- Description HATU, first prepared by Louis A. Carpino in 1993, is widely used in carboxylic acid amidation reactions. It acts as a facilitator of amide bond generation by activating the carboxyl group.
- Chemical Properties White crystalline to off-white powder
-
Uses
HATU[148893-10-1] is a coupling reagent and used as an additive in peptide synthesis. It is also involved efficiently to speed up the coupling process and reduces the loss of chiral integrity.
Reagent for: Synthesis of Aurora A kinase inhibitors, HPLC assay to determine D- and L- acid enantiomers in human plasma, Amide bond formation reactions.
Catalyst for: Selective acylation, Selecocyclization-oxidation deselenation sequence. -
Reactions
HATU is a very promising coupling agent for chemical protein synthesis.
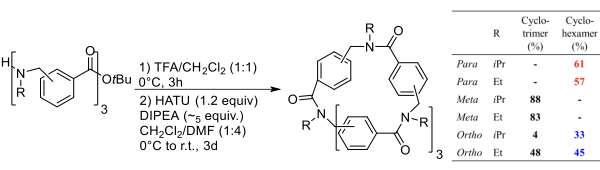
This strategy was exploited to prepare macrocycles from the trimeric linear arylopeptoids (ortho-, meta-, and para-) containing isopropyl or ethyl side chains, synthesized as described by Hjelmgaard et al. The cyclization procedure reported for α,β-cyclopeptoids was applied. The linear arylopeptoids were cyclized in the presence of HATU and DIPEA in CH?Cl?/DMF (4:1) after the deprotection of the tert-butyl group in TFA/CH?Cl?.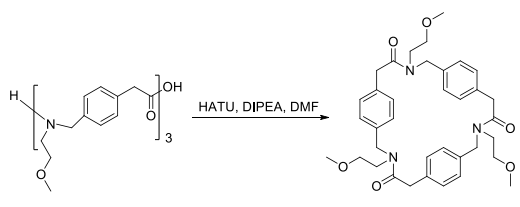
After the synthesis of the Fmoc-protected monomers, the oligomers were synthesized on the 2-chlorotrityl resin with excellent yield of coupling (>98%). The trimers and tetramers of the different isomers were synthesized in good overall yield (60-84%). Then, the crude oligomers were cyclized in DMF in the presence of HATU and DIPEA in high dilution (3 × 10?3 M) to furnish the cyclized trimers and tetramers in good yields ranging between 32% and 72%. - reaction suitability reaction type: Coupling Reactions
-
Synthesis
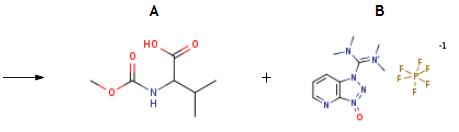
The synthesis of HATU is as follows:The resulting residue treated with 2-Methoxycarbonylamino-3-methyl-butyric acid (60 mg, 0.343 mmol) and HATU (130 mg, 0.343 mmol), suspended in DMF (3 mL) and cooled to 0° C. DIPEA (0.272 mL, 1.56 mmol) was added dropwise. After stirring for 4 h, NaOH (5M in H2O, 0.300 mL, 1.5 mmol) was added. This mixture was stirred for 3 h then diluted with EtOAc and washed with 1 M LiOH (2*) then brine. The organic phase was dried over MgSO4, filtered and concentrated. The crude residue was then purified by HPLC to afford the title compound (53 mg, 44%).
-
References
[1] CHAWLA P A, SHOME A, JHA K T. Hexafluorophosphate Azabenzotriazole Tetramethyl Uronium (HATU): A Unique Cross-Coupling Reagent[J]. SynOpen, 2023, 66 2: 0. DOI:10.1055/s-0042-1751499.
[2] LOUIS A. CARPINO. Comparison of the Effects of 5- and 6-HOAt on Model Peptide Coupling Reactions Relative to the Cases for the 4- and 7-Isomers?,?[J]. Organic Letters, 2000, 2 15: 2253-2256. DOI:10.1021/ol006013z.
HATU Preparation Products And Raw materials
Raw materials
Preparation Products
Global(699)Suppliers
-
Supplier:
Zhengzhou Anbu Chem Co.,Ltd
- Tel:+86-0371-88006763;<br/>+8615988602810
- Email:sales@anbuchem.com
- Country:China
- ProdList:2998
- Advantage:58
-
Supplier:
LEAP CHEM CO., LTD.
- Tel:+86-852-30606658
- Email:market18@leapchem.com
- Country:China
- ProdList:24727
- Advantage:58
-
Supplier:
Watson Biotechnology Co.,Ltd
- Tel:+86-18186686046<br/>+86-18186686046
- Email:sales01@watsonbiotech.cn
- Country:China
- ProdList:5849
- Advantage:58
-
Supplier:
Jiangsu Vcan Advanced Material Co., Ltd.
- Tel:+86-17306299870<br/>+86-18652789875
- Email:vcanam@vcanam.com
- Country:China
- ProdList:38
- Advantage:58
-
Supplier:
Hebei Chuanghai Biotechnology Co., Ltd
- Tel: +8615531157085
- Email:abby@chuanghaibio.com
- Country:China
- ProdList:8808
- Advantage:58
-
Supplier:
Wuhan Fortuna Chemical Co., Ltd
- Tel:+86-027-59207850
- Email:info@fortunachem.com
- Country:China
- ProdList:5971
- Advantage:58
-
Supplier:
Hebei Chuanghai Biotechnology Co,.LTD
- Tel: +86-13131129325
- Email:sales1@chuanghaibio.com
- Country:China
- ProdList:5869
- Advantage:58
-
Supplier:
XINXIANG RUNYU MATERIAL CO., LTD.
- Tel:+86-13592593621;<br/>+8613592593621
- Email:sales@runvmat.com
- Country:China
- ProdList:408
- Advantage:58
-
Supplier:
Hebei Fengjia New Material Co., Ltd
- Tel:+86-0311-87836622<br/>+86-18712993135
- Email:sales01@tairunfaz.com
- Country:China
- ProdList:8051
- Advantage:58
-
Supplier:
Henan Bao Enluo International TradeCo.,LTD
- Tel:+86-17331933971<br/>+86-17331933971
- Email:deasea125996@gmail.com
- Country:China
- ProdList:2472
- Advantage:58
Related articles
148893-10-1, HATURelated Search:
- Ammonium hexafluorophosphate Potassium hexafluorophosphate Lithium hexafluorophosphate Ribavirin 1,2,4-Triazole 5-Methyl-1H-benzotriazole Hexafluorophosphoric acid 1H-Benzotriazole HBTU N-Benzyl-DL-serine Methyl Ester Dess-Martin periodinane 3-PHENYL-1H-PYRAZOLE-4-CARBALDEHYDE N-Bromosuccinimide AM2201 Ethyl (R)-(-)-4-cyano-3-hydroxybutyate Nitrogen Azobenzene HOAt
- 148893-10-1
- intermediate
- Peptide
- Peptide coupling agents
- Amino Acid Derivatives
- Pharmaceutical Intermediates
- PROTECTED AMINO ACID & PEPTIDES
- Coupling Reagent
- Miscellaneous Reagents
- Peptide Coupling Reagents
- HATU
- carboxylic ester
- peptides
- 抑制剂
- 化工助剂
- 其它原料及中间体
- 多肽缩合剂
- 高端化学
- 原料药
- 生物化工-氨基酸及其衍生物
- 化学试剂
- 偶联剂及保护剂
- 化学原料
- 化工中间体
- 通用生化试剂-氨基酸
- 医药原料
- 化工原料
- 中间体-有机中间体
- 有机醚
- 基础原料
- 医用原料
- 多肽试剂-缩合剂
- 生化试剂-氨基酸类
- 缩合剂
- 催化剂
- 苯并杂环
- 氨基酸
- 医药中间体
- 耦合试剂
- 中间体
- 缩合试剂
- 合成试剂
- 耦合
- 保护氨基酸及多肽类产品
- 多肽
- 保护氨基酸
- 肽键缩合剂
- 多肽偶联剂
- 杂环类
- 氨基酸类
- New Products for Chemical Synthesis May/June 2007
- Phosphonium/Uronium/Formamidinium
- Coupling
- Synthetic Reagents
- C10H15N6O.F6P
- C10H15F6N6OP
- C10H15N6O
- C10H15N6OPF6