ChemicalBook > CAS DataBase List > TRIMETHYLENE DI(THIOTOSYLATE)
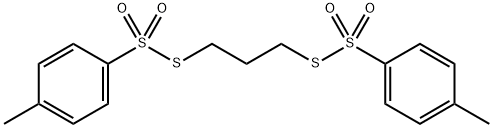
TRIMETHYLENE DI(THIOTOSYLATE)
TRIMETHYLENE DI(THIOTOSYLATE)
- CAS No.3866-79-3
- Chemical Name:TRIMETHYLENE DI(THIOTOSYLATE)
- CBNumber:CB0100135
- Molecular Formula:C17H20O4S4
- Formula Weight:416.58
- MOL File:3866-79-3.mol
TRIMETHYLENE DI(THIOTOSYLATE) Property
- Melting point 64-67 °C
- Boiling point 592.3±60.0 °C(Predicted)
- Density 1.340±0.06 g/cm3(Predicted)
- form powder to crystal
- color White to Almost white
- BRN 2020157
- CAS DataBase Reference 3866-79-3
- UNSPSC Code 12352100
- NACRES NA.22
Safety
- Safety Statements :22-24/25
- WGK Germany :3
- HS Code :2930.90.2900
-
Symbol(GHS)

- Signal wordWarning
- Hazard statements H302-H315-H319-H335
- Precautionary statements P261-P305+P351+P338
TRIMETHYLENE DI(THIOTOSYLATE) Price
More Price(2)
- Brand: TCI Chemicals (India)
- Product number: D2390
- Product name : Trimethylene Di(thiotosylate) [Protecting Reagent for Active Methylene]
- Purity:
- Packaging: 5G
- Price: ₹5300
- Updated: 2022/05/26
- Buy: Buy
- Brand: TCI Chemicals (India)
- Product number: D2390
- Product name : Trimethylene Di(thiotosylate) [Protecting Reagent for Active Methylene]
- Purity:
- Packaging: 25G
- Price: ₹8800
- Updated: 2022/05/26
- Buy: Buy
TRIMETHYLENE DI(THIOTOSYLATE) Chemical Properties,Usage,Production
- Uses Trimethylene Di(thiotosylate) is used as a reactant in the enantioselective total synthesis of (-)-O-methylpallidinine, which is a naturally occurring morphinan alkaloid.
-
Reactions
1,3-Propanedithiol Bis(p-toluenesulfonate) could be used in the below reactions:
dithianylation and dithiolanylation of methylene groups adjacent to carbonyl; α,α-dithianyl cycloalkanones undergo oxidative ring scission; oxidation of ketones to 1,2-diketones can be achieved by α,α-dithianylation or dithiolanation of ketones followed by hydrolysis. - Synthesis Treatment of potassium thiotosylate, itself made by the reaction of p-Toluenesulfonyl Chloride with Potassium Hydrogen Sulfide, with 1,3-dibromopropane or 1,2-dibromoethane in the presence of Potassium Iodide affords 1,3-propanedithiol bis(p-toluenesulfonate) and 1,2-ethanedithiol bis(p-toluenesulfonate), respectively.
TRIMETHYLENE DI(THIOTOSYLATE) Preparation Products And Raw materials
Raw materials
Preparation Products
Global(70)Suppliers
-
Supplier:
Hebei Chuanghai Biotechnology Co., Ltd
- Tel: +8615531157085
- Email:abby@chuanghaibio.com
- Country:China
- ProdList:8808
- Advantage:58
-
Supplier:
Shaanxi Dideu Medichem Co. Ltd
- Tel:+86-029-81138252<br/>+86-18789408387
- Email:1057@dideu.com
- Country:China
- ProdList:3922
- Advantage:58
-
Supplier:
Hebei Mujin Biotechnology Co.,Ltd
- Tel:+86 13288715578<br/>+8613288715578
- Email:sales@hbmojin.com
- Country:China
- ProdList:12809
- Advantage:58
-
Supplier:
career henan chemical co
- Tel:+86-0371-86658258<br/>+8613203830695
- Email:sales@coreychem.com
- Country:China
- ProdList:29858
- Advantage:58
-
Supplier:
Hefei TNJ Chemical Industry Co.,Ltd.
- Tel:+86-0551-65418671<br/>+8618949823763
- Email:sales@tnjchem.com
- Country:China
- ProdList:34563
- Advantage:58
-
Supplier:
Coresyn Pharmatech Co., Ltd.
- Tel:+86-571-86626709<br/>+86-18157142896
- Email:cbc@coresyn.com
- Country:China
- ProdList:9984
- Advantage:58
-
Supplier:
Zibo Hangyu Biotechnology Development Co., Ltd
- Tel:+86-0533-2185556<br/>+8615965530500
- Email:nickzhang@hangyubiotech.com
- Country:China
- ProdList:10983
- Advantage:58
-
Supplier:
Aladdin Scientific
- Tel:
- Email:tp@aladdinsci.com
- Country:United States
- ProdList:52924
- Advantage:58
-
Supplier:
Qingdao Dexin Chemical Co., Ltd
- Tel:+86-15553333686<br/>+86-15553333686
- Email:15553333686@qq.com
- Country:China
- ProdList:3113
- Advantage:58
-
Supplier:
Hebei Chuanghai Biotechnology Co., Ltd
- Tel:+86-15350571055<br/>+86-15350571055
- Email:Sibel@chuanghaibio.com
- Country:China
- ProdList:6086
- Advantage:58
3866-79-3, TRIMETHYLENE DI(THIOTOSYLATE)Related Search:
- Styrene Methyl propyl disulfide methyl methanethiosulfinate Propyl Methanethiosulfonate S-METHYL 4-METHYLBENZENETHIOSULFONATE TRIMETHYLENE DI(THIOTOSYLATE) 1,3-Propanediyl Bismethanethiosulfonate ETHYL METHANETHIOSULFONATE S-Methyl methanethiolsulfonate METHYL PHENYL DISULFIDE PHENYLETHYLDISULFIDE 1,3-Dimercaptopropane
- BuildingBlocks
- blocks
- Sulfur Compounds (for Synthesis)
- Protection & Derivatization Reagents (for Synthesis)
- Synthetic Organic Chemistry
- Tosylates
- Sulfur Compounds
- Organic Building Blocks
- Chemical Synthesis
- Building Blocks
- 其他生化试剂
- 有机砌块
- 亚磺酸盐
- 磺酸
- Tosylates
- Sulfur Compounds
- Organic Building Blocks
- Building Blocks
- Sulfur Compounds (for Synthesis)
- Protection & Derivatization Reagents (for Synthesis)
- C17H20O4S4
- CH3C6H4SO2SCH2CH2CH2SSO2C6H4CH3
- -三甲烯(P-甲苯硫代磺酸盐)
- S,S'-三甲烯(|P|-甲苯硫代磺酸盐)
- 1,3-双(4-甲基苯磺酰硫基)丙烷
- 三亚甲基二(硫代对甲苯磺酸酯)[活泼亚甲基的保护试剂]
- 三甲烯二(甲苯硫代磺酸盐)
- 1,3-二(对甲苯磺酰基硫代)丙烷
- 三亚甲基二(硫代对甲苯磺酸酯)
- 4-甲基苯硫代磺酸 S1,S1'-1,3-丙烷二基酯
- s,s'-三甲烯(p-甲苯硫代磺酸盐)
- 3866-79-3
- s,s'
- <i>S</i>,<i>S</i>′-Trimethylene di(<i>p</i>-toluenethiosulfonate)
- TrimethyleneDi(thiotosylate)[ProtectingReagentforActiveMethylene]>
- 1,3-bis(p-toluenesulfonylthio)propane
- 4-Methylbenzenesulfonothioic acid S1,S1'-1,3-propanediyl ester
- 1,3-Propanedithiol ditosylate
- 1,3-Bis(4-methylbenzenesulfonylthio)propane
- 1-methyl-4-[3-[(4-methylphenyl)sulfonylthio]propylthio]sulfonyl-benzene
- 1-methyl-4-[3-(4-methylphenyl)sulfonylsulfanylpropylsulfanylsulfonyl]benzene
- 1,3-Di(p-tosylthio)propane 1,3-Propanediyl Di(thiotosylate) Trimethylene Bis(p-toluenethiosulfonate)
- Benzenesulfonothioicacid, 4-methyl-, S1,S1'-1,3-propanediyl ester
- Trimethylene di-p-toluenethiosulfonate
- NSC 82472
- p-Toluenesulfonothioic acid S-[3-[(4-methylphenyl)sulfonylthio]propyl] ester
- 1,3-Propanediylbis(tosyl sulfide)
- 1,3-Propanedithiolebis(p-toluenesulfonate)
- 1,3-Di(p-tosylthio)propane, 1,3-Propanediyl di(thiotosylate), Trimethylene di(thiotosylate)
- Benzenesulfonothioic acid, 4-methyl-, S,S-1,3-propanediyl ester
- S,S'-Trimethylene di(p-toluenethiosulfonate)
- Trimethylene Di(thiotosylate) [Protecting Reagent for Active Methylene]
- TRIMETHYLENE DI(THIOTOSYLATE)
- TRIMETHYLENE BIS(P-TOLUENETHIOSULFONATE)
- 1,3-PROPANEDIYL DI(THIOTOSYLATE)
- 1,3-DI(P-TOSYLTHIO)PROPANE