lepidolite
- Product Namelepidolite
- CAS1317-64-2
- CBNumberCB9945379
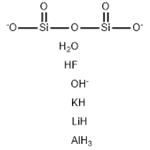
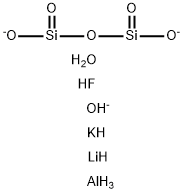
- MFAlFH9KLiO7Si2(-3)
- MW269.25
- MOL File1317-64-2.mol
lepidolite Chemical Properties,Usage,Production
Description
Before spodumene became the sole ore for the recovery of lithium for chemical use, lepidolite was used as a lithium source.One process for recovery of lithium from lepidolite used sulfuric acid in a procedure similar to that used by the Lithium Corporation of America for spodumene. A more recent method involved heating a mixture of lepidolite and potassium sulfate to just below the melting point and extracting the mixture with water to recover a solution of lithium sulfate.

Chemical Properties
Lepidolite is named after the Greek word Lepidos, which means "scale". This is due to the typical formation of the mineral in scale-like clusters. Lepidolite contains between 1.23% to 5.90% lithium oxide and has a monoclinic crystal system with dimensions a=5.3, b=9.2, c=10.2, and β=100°. The crystal symmetry is m or 2/m, with pseudo-hexagonal plates forming fine scale-like clusters. Its attractive light purple color is combined with a glass luster, a hardness of 2-3, and a relative density of 2.8-2.9. There are over 150 minerals containing lithium, but only 30 of these are independent lithium minerals. The most important of these minerals is spodumene, followed by lepidolite.Agricultural Uses
Lepidolite, a variety of mica, is a fluorosilicate of potassium, lithium and aluminum found in pegmatites. Generally, rubidium occurs as an impurity.Preparation Products And Raw materials
lepidolite Supplier
Global(7)Suppliers
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| +86-29-81148696 +86-15536356810 |
1022@dideu.com | China | 3882 | 58 | |
| 18871490254 | linda@hubeijusheng.com | CHINA | 28172 | 58 | |
| +86-023-6139-8061 +86-86-13650506873 |
sales@chemdad.com | China | 39894 | 58 | |
| +86-0512-83500002 +8615195660023 |
sales@senfeida.com | China | 23046 | 58 | |
| +8618950047208 | ellena@amitychem.com | China | 43416 | 58 | |
| +86-21-5877 1921 | info@chemofchina.com | China | 9653 | 55 |
Related articles
Geologic Occurrence and uses of Lepidolite
Lepidolite is the name of a rare lithium-rich mica mineral that is usually pink, red, or purple in color.
Dec 4,2019
1317-64-2, lepidoliteRelated Search
PROMPT×
PROMPT
The What'sApp is temporarily not supported in mainland China
The What'sApp is temporarily not supported in mainland China
Cancel
Determine