Chemical Properties
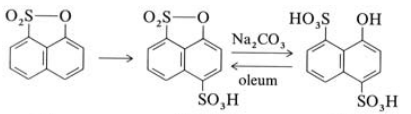
1-Hydroxynaphthalene-4,8-disulfonic acid [117-56-6]. (4-hydroxynaphthalene-1,5- disulfonicacid),Schollkopf ’s acid, deltaacid, oxy Chicago acid, C10H8O7S2, Mr 304.3: the sodium saltis readily solubleinwater, butthe barium saltis only sparingly soluble.Cold5 % oleumdehydrates 1-Hydroxynaphthalene-4,8-disulfonic acid to the sultone, whereas with hot 25 % oleum it undergoes sulfonation to give 1-hydroxynaphthalene-2,4,8-trisulfonic acid. Amination (Bucherer) gives 1-aminonaphthalene-4,8-disulfonic acid and caustic fusion gives 1,8-dihydroxynaphthalene-4-sulfonic acid. Diazo coupling and nitrosation take place in the 2-position.
Production Methods
1-Hydroxynaphthalene-8-sulfonic acid-1,8-sultone is heated with sulfuric acid at 80 – 90℃. The reaction mixture is then quenched by pouring into concentrated brine, whereby the sodium salt of 82 crystallizes. After isolation, this intermediate is hydrolyzed by heating with aqueous sodium carbonate and the product is isolated as its sodium salt after cooling.