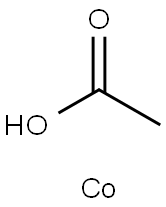
Chemical Properties
dark green, very hygroscopic powder(s) or green octahedral crystal(s); used as a catalyst for cumene hydroperoxide [KIR79] [MER06]
Uses
Catalyst for cumene hydroperoxide decomposition.
Application
Used as one-electron oxidizing agent for aromatic and aliphatic hydrocarbons, for oxidative halogenation, oxidation of alcohols and acids.
Synthesis
Cobalt triacetate is prepared by involves oxidation of Cobalt(II) Acetate in acetic acid solution. The various oxidants used for this purpose are ozone, peracid, peroxide, and oxygen. An electrochemical procedure for the generation of Co(OAc)3 in solution is also available.