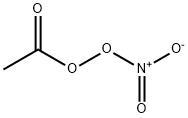
Uses
Peroxyacetyl Nitrate is a convenient source of acetyl peroxy radicals; it epoxidizes alkenes; oxidizes aldehydes, thiols and sulfides; acetylates primary amines.
Safety Profile
Poison by inhalation. A human eye irritant. Mutation data reported. Extremely explosive. When heated to decomposition it emits toxic fumes of NOx.
Synthesis
To a stirred mixture of Peracetic Acid (5 mmol) and 10-15 mL of dry pentane maintained at -5 to 0 °C is added 90% Nitric Acid (1 equiv) and Sulfur Trioxide (30% SO3 in H2SO4, 1 equiv). The reaction mixture is stirred at -5 °C for 3 h and then treated with ice water. The organic layer is dried over MgSO4 at -20 °C. The yield of unpurified Peroxyacetyl Nitrate in pentane solution is 39%.
Precautions
Explosions occur when pure PAN is condensed as a liquid. PAN (peroxyacetyl nitrate) is thermally unstable above 0 °C. Purified PAN is stored as an N2-diluted gas at 1000 ppm, 100 psi in gas cylinders at 0 °C. Use in a well-ventilated fume hood.