Manufacturing Process
A mixture of 11.4 parts of 5-methyloxazolidine-2,4-dione and 15 parts
anhydrous potassium carbonate in 150 parts of dry acetone is stirred for 0.5
hour. 15 parts of allyl bromide are then added and the mixture boiled under
reflux with stirring for 4 hours. After cooling and filtering, the solvent and any
unchanged allyl bromide are removed by distillation. The residue is extracted
with ether end the extract washed with saturated aqueous sodium bicarbonate
until the subsequent water-washings are either neutral or just alkaline to
litmus paper. The solvent is then distilled and the residue fractionated when 3-
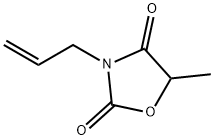
allyl-5-methyloxazolidine-2,4-dione is obtained in 65% yield as a colorless oil,
BP: 88°-90°C/1.8 mm, nd20 = 1.4710.
The dry sodium salt of 5-methyloxazolidine-2,4-dione, obtained from 4.6 parts
of sodium, 100 parts of ethanol, 12 parts of urea and 23.6 parts of ethyl
lactate, is suspended in 100 parts of dry benzene and 30.25 parts of allyl
bromide are added. The mixture is boiled under reflux for 20 hours and the
benzene decanted or filtered from any solid. The solution is washed with saturated aqueous sodium bicarbonate until the subsequent water-washings
are neutral or just alkaline to litmus paper. The dried benzene solution is then
distilled to remove solvent and the residue fractionated, when 3-allyl-5-
methylosazolidine-2,4-dione is obtained in 27% yield as a colorless oil, BP:
89°-90°C/1 mm, nd20 = 1.4712.
The dry sodium salt of 5-methyloxazolidine-2,4-dione obtained as above is
mixed with 100 parts of dry dioxane and 31.25 parts of allyl bromide are
added. After boiling under reflex for 24 hours, decanting the solution and
distilling off the dioxane under reduced pressure, the residue is dissolved in
ether and the ethereal extract mashed with aqueous sodium bicarbonate as
above. Fractionation of the residue after removing the olvent furnishes 3-allyl-
5-methyloxazolidine-2,4-dione in 48.5% yield as a colorless oil; BP: 94°-
95°C/11.75 mm; nd20 = 1.4710.