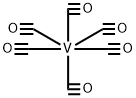
Chemical Properties
Bluish green crystalline solid; decomposeson heating at 70°C (158°F); insoluble inwater and alcohol; low solubility in hexaneand other saturated hydrocarbons, but solublein most other organic solvents (solution turnsyellow).
Uses
Hexacarbonylvanadium is used as a catalyst in isomerization andhydrogenation reactions.
Health Hazard
There is no report on its toxicity in the publishedliterature. However, this substance,like most other metal carbonyls, shouldbe treated as a potential health hazard asit can produce highly toxic decompositionproducts, carbon monoxide, and vanadiumoxides. Skin contact can cause irritation.
Fire Hazard
Vanadium hexacarbonyl is a pyrophoric compound.
It can explode on heating.