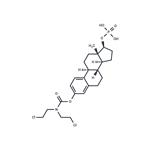
![estra-1,3,5(10)-triene-3,17beta-diol 3-[bis(2-chloroethyl)carbamate] 17-(dihydrogen phosphate) Structure](https://www.chemicalbook.com/CAS/GIF/4891-15-0.gif)
estra-1,3,5(10)-triene-3,17beta-diol 3-[bis(2-chloroethyl)carbamate] 17-(dihydrogen phosphate)
- Product Nameestra-1,3,5(10)-triene-3,17beta-diol 3-[bis(2-chloroethyl)carbamate] 17-(dihydrogen phosphate)
- CAS4891-15-0
- CBNumberCB9876255
- MFC23H32Cl2NO6P
- MW520.383041
- EINECS225-512-3
- MDL NumberMFCD00869450
- MOL File4891-15-0.mol
- MSDS FileSDS
Chemical Properties
| Melting point | 155 °C |
| Boiling point | 661.2±65.0 °C(Predicted) |
| Density | 1.40±0.1 g/cm3(Predicted) |
| pka | 1.88±0.10(Predicted) |
| FDA UNII | MUZ9585Y7B |