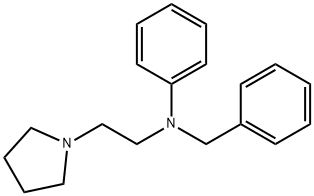
histapyrrodine
- Product Namehistapyrrodine
- CAS493-80-1
- CBNumberCB9875339
- MFC19H24N2
- MW280.41
- EINECS207-781-9
- MDL NumberMFCD00865692
- MOL File493-80-1.mol
- MSDS FileSDS
Chemical Properties
| Boiling point | bp1 198-205° |
| pka | pKa 2.99± 0.12;7.95 ± 0.02(H2O,t undefined,I=0.30(NaCl)) (Uncertain) |
| FDA UNII | 0FYM61NG4D |
| ATC code | R06AC02,R06AC52 |