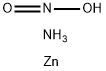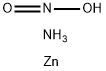Definition
White powder. Strong oxidizing agent.
General Description
A white powder. Combustible and readily ignited. Easily ignites if contaminated with combustible materials. May explode under exposure to heat or fire. Produces toxic oxides of nitrogen during combustion.
Air & Water Reactions
Water soluble.
Reactivity Profile
A violent explosion occurs if an ammonium salt is melted with a nitrite salt [Von Schwartz 1918. p. 299]. A mixture of potassium cyanide and nitrite salts may cause an explosion [Pieters 1957. p. 30]. Reacts with acids to form toxic nitrogen dioxide gas.
Health Hazard
Inhalation, ingestion or contact (skin, eyes) with vapors or substance may cause severe injury, burns or death. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may cause pollution.
Fire Hazard
These substances will accelerate burning when involved in a fire. Some may decompose explosively when heated or involved in a fire. May explode from heat or contamination. Some will react explosively with hydrocarbons (fuels). May ignite combustibles (wood, paper, oil, clothing, etc.). Containers may explode when heated. Runoff may create fire or explosion hazard.