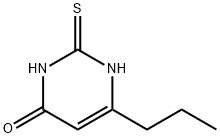
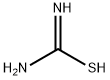
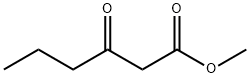
Description
Propylthiouracil (PTU) is a thioamide antithyroid agent. It inhibits thyroid peroxidase activity in rat and monkey thyroid microsomes (IC
50s = 0.081 and 4.1 μM, respectively). PTU (30 mg/kg) increases thyroid weight and serum thyroid stimulating hormone levels and decreases serum 3,5,3''-triiodothyronine and thyroxine levels in rats. Sensitivity to the bitter taste of PTU is genetically mediated and is associated with increased sensitivity to other sweet and bitter compounds. Formulations containing propylthiouracil have been used in the treatment of Graves'' disease and hyperthyroidism.
Chemical Properties
White crystalline solid or powder. Odorless.
Bitter taste.
Chemical Properties
White Crystalline Powder
Uses
Antihyperthyroid. Has been used to promote fattening. This substance is reasonably anticipated to be a human carcinogen.
Definition
ChEBI: A pyrimidinethione consisting of uracil in which the 2-oxo group is substituted by a thio group and the hydrogen at position 6 is substituted by a propyl group.
General Description
Odorless white crystalline powder of starch-like appearance. Bitter taste. Saturated solution is neutral or slightly acid to litmus.
General Description
Propylthiouracil, 6-propyl-2-thiouracil (Propacil), is a stable, white, crystalline powderwith a bitter taste. It is slightly soluble in water but readilysoluble in alkaline solutions (salt formation).
Air & Water Reactions
Sensitive to light. May be sensitive to prolonged exposure to air. Insoluble in water.
Reactivity Profile
Propylthiouracil is incompatible with strong oxidizing agents, strong acids and strong bases. Forms complexes with divalent metals. Reacts with sulfhydryl-oxidizing agents . When reduced will produce hydrogen sulfide.
Hazard
Possible carcinogen.
Fire Hazard
Flash point data for Propylthiouracil are not available; however Propylthiouracil is probably combustible.
Biochem/physiol Actions
6-Propyl-2-thiouracil (6-PTU), a potential inhibitor of D1 iodothyronine deiodinase, is involved in the deiodination of iodothyronines. It is an uncompetitive inhibitor of iodothyronine substrates.
Mechanism of action
This drug has a pronounced thyrostatic effect and causes reduced thyroxine synthesis in
the thyroid gland. It inhibits the process of iodination of thyroglobulin, reduces formation
of the active form of iodine in the thyroid gland, and blocks the peroxidase system.
Propylthiouracil is used for hyperthyrosis, thyrotoxic crises, and on thyrodectomia.
Synonyms of this drug are propycil and tireostat.
Clinical Use
Propylthiouracil is useful in the treatment of hyperthyroidism.There is a delay in appearance of its effects because propylthiouracildoes not interfere with the activity of thyroid hormonesalready formed and stored in the thyroid gland. Thislag period may vary from several days to weeks, dependingon the condition of the patient. The need for three equallyspaced doses during a 24-hour period is often stressed, butevidence now indicates that a single daily dose is as effectiveas multiple daily doses in the treatment of most hyperthyroidpatients.
Safety Profile
Confirmed carcinogen with experimental carcinogenic, neoplastigenic, and tumorigenic data. Poison by intraperitoneal route. Moderately toxic by ingestion. Human systemic effects: agranulocytosis, hepatitis, jaundice. Human teratogenic effects by ingestion:
developmental abnormalities of the endocrine system and changes in newborn viability. Human and experimental teratogenic and reproductive effects. Human mutation data reported. When heated to decomposition it emits very toxic fumes of SO, and NO,. See also MERCAPTANS.
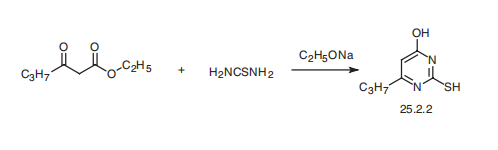
Synthesis
Propylthiouracil, 6-propyl-2-thio-2,4-(1H,3H)-pyrimidindione (25.2.2),
is synthesized by condensating ethyl butyroacetate with thiourea in the presence of sodium
ethoxide.
Potential Exposure
Medication (antihyperthyroid; thyroid
inhibitor) with human and veterinary applications.
Carcinogenicity
Propylthiouracil is reasonably anticipated to be a human carcinogenbased on sufficient evidence of carcinogenicity from studies in experimental animals.
Metabolism
Propylthiouracil undergoes rapid first-pass metabolism
in the liver, and is mainly excreted in the urine as the
glucuronic acid conjugate, with very little excreted as
unchanged drug.
Shipping
UN3249 Medicine, solid, toxic, n.o.s., Hazard
Class: 6.1; Labels: 6.1-Poisonous materials.
Purification Methods
Purify propacil by recrystallisation from H2O (soluble in 900 parts at 20o, and 100 parts at 100o). UV, MeOH: max 277nm. [Anderson et al. J Am Chem Soc 67 2197 1945, Vanderhaegue Bull Soc Chim Belg 59 689 1950, Beilstein 24 III/IV 1333.]
Incompatibilities
This chemical is probably combustible;
it’s dust may form explosive mixture with air. Incompatible
with oxidizers (chlorates, nitrates, peroxides, permanga-
nates, perchlorates, chlorine, bromine, fluorine, etc.); con-
tact may cause fires or explosions. Keep away from
alkaline materials, strong bases, strong acids, oxoacids,
epoxides. Forms complexes with divalent metals. Reacts
with sulfhydryl-oxidizing agents . Sensitive to light and
may be sensitive to air.
Waste Disposal
It is inappropriate and possi-
bly dangerous to the environment to dispose of expired
or waste pharmaceuticals by flushing them down the toilet
or discarding to trash. Household quantities of expired or
waste pharmaceuticals may be mixed with wet cat litter or
coffee grounds, double-bagged in plastic, discard in trash.
Larger quantities shall carefully take into consideration
applicable DEA, EPA, and FDA regulations. If possible
return the pharmaceutical to the manufacturer for proper
disposal being careful to properly label and securely pack-
age the material. Alternatively, the waste pharmaceutical
shall be labeled, securely packaged and transported by
a state licensed medical waste contractor to dispose by burial in a licensed hazardous or toxic waste landfill or
incinerator.