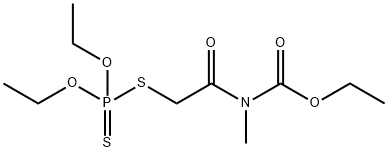
Description
Mecarbam is a pale yellow to light brown oil, bp
144 ?C/0.02 mm Hg. It is slightly soluble in water (<0.1%
at 25 ?C) and miscible with most organic solvents except
alkanes. Log Kow = 2.6. It is readily hydrolyzed in aqueous
media below pH 3.
Chemical Properties
Yellow oil.
Uses
Mecarbam is an organothiophosphate acaricides used to control powdery mildew of cucumbers.
Uses
Mecarbam is an insecticide with some systemic activity which is
used to control sucking insects and mites in fruit trees and plant hoppers
and leaf miners in rice. Additional uses are the control of whitefly, leafhoppers
and thrips on rice and root flies on vegetables.
Definition
ChEBI: An organic thiophosphate that is O,O-diethyl hydrogen phosphorodithioate in which the hydrogen attached to a sulfur is replaced by a 2-[(ethoxycarbonyl)(methyl)amino]-2-oxoethyl group.
Hazard
Highly toxic, cholinesterase inhibitor. Use
may be restricted.
Metabolic pathway
Mecarbam in a close chemical analogue of dimethoate and it is photolytically
decarboxyethylated to the diethyl analogue of dimethoate. The main
route of hydrolytic decomposition in moderately basic solution is via
attack on the S-methylene carbon atom rather than on phosphorus or the
carbamoyl group, followed by cleavage of the S-C bond yielding O,Odiethyl
phosphorodithioate as the main product. Mecarbam oxon has
been reported as a metabolite in plants and animals but few detailed
studies have been reported on the metabolic or environmental fate of
mecarbam.
Metabolism
In mammals, mecarbam
is rapidly metabolized by hydrolysis, oxidative desulfuration
to the oxon, and degradation of the carbamoyl moiety.
O-Deethylation also takes place to a minor extent. In soil,
it persists for 4–6 weeks.
Toxicity evaluation
Acute oral LD
50 for
rats is 35–53 mg/kg. Inhalation LC50 (6 h) for rats is
0.7 mg/L air. ADI is 0.002 mg/kg.
Degradation
The rate of hydrolysis and the nature of the products when mecarbam
was dissolved in buffered water at pH values between 2.2 and 10 were
reported by Lynch et al. (1981). The analysis of mecarbam and its metabolites
was by GC-MS with and without methylation by diazomethane and
by TLC co-chromatography with reference materials and included the use
of chromogenic sprays to differentiate between phosphorothioates (P=S)
and phosphates (P=O) . Mecarbam was stable in acid media but was
hydrolysed in alkaline solutions with a half-life of about 44 hours at pH
9.2. The principal product of base hydrolysis was O,O-diethyl phosphorodithoate
(2), indicating that mecarbam was mainly hydrolysed by
nucleophdic attack on the S-methylene carbon atom followed by cleavage
of the S-C bond. There was evidence that O,O-diethyl phosphorodithioate
(2) was hydrolysed further to give the des-ethyl compound, O-ethyl
phosphorodithioate (3). When mecarbam was hydrolysed under much
more basic conditions (0.5 M aqueous potassium hydroxide) the main
routes of hydrolysis were attack by OH- on the amido carbon atom
followed by cleavage of the C-N bond to give S-carboxymethyl O,Odiethyl
phosphorodithioate (4) and attack on the phosphorus atom followed
by cleavage of the P-S bond to give O,O-diethyl phosphorothioate
(5). Initial hydrolysis on the carboethoxy (carbamoyl) group was not
observed and nor was reaction product 2 detected, indicating that initial
attack on the phosphorus and carbonyl centres was faster in strongly basic
solution (Hudson et al., 1991).
The main product of photolysis when mecarbam was absorbed on
a filter paper and exposed to 254 nm UV light from a mercury vapour
lamp was the decarboxyethylated compound O,O-diethyl S-methylcarbamoylmethyl
phosphorodithioate (6) (the diethyl analogue of
dimethoate). The rate of &us reaction was potentiated by the surface of
certain leaves, with dock leaves being particularly effective.O,O-Diethyl
phosphorodithioate (2) was also detected in trace amounts (Lynch et al.,1981). Proposed routes for the photolysis and base-catalysed hydrolysis
of mecarbam are shown in Scheme 1.