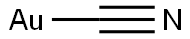
GOLD(I) CYANIDE
- Product NameGOLD(I) CYANIDE
- CAS506-65-0
- CBNumberCB9746055
- MFCAuN
- MW222.98
- EINECS208-049-1
- MDL NumberMFCD00003437
- MOL File506-65-0.mol
Chemical Properties
| Melting point | ~50°C |
| Density | 7,12 g/cm3 |
| solubility | insoluble in H2O, ethanol, ethyl ether,dilute acid solutions |
| form | Powder |
| Specific Gravity | 7.14 |
| color | Yellow |
| Water Solubility | Slightly soluble in water. |
| Sensitive | Moisture Sensitive |
| Merck | 14,4516 |
| Exposure limits | NIOSH: IDLH 25 mg/m3 |
| CAS DataBase Reference | 506-65-0(CAS DataBase Reference) |
| FDA UNII | 03I25JDS1H |
| EPA Substance Registry System | Gold cyanide (Au(CN)) (506-65-0) |
Safety
| Symbol(GHS) |
  
|
|||||||||
| Signal word | Danger | |||||||||
| Hazard statements | H300+H310+H330-H410-H290-H301-H310-H315-H318-H330-H400 | |||||||||
| Precautionary statements | P260-P264-P273-P280-P284-P301+P310-P301+P310a-P304+P340-P305+P351+P338-P320-P330-P405-P501a | |||||||||
| Hazard Codes | T+,N | |||||||||
| Risk Statements | 26/27/28-32-50/53 | |||||||||
| Safety Statements | 7-28-29-60-61-45 | |||||||||
| RIDADR | 1588 | |||||||||
| WGK Germany | 3 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 6.1 | |||||||||
| PackingGroup | II | |||||||||
| HS Code | 28433000 | |||||||||
| NFPA 704: |
|
