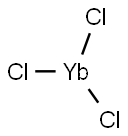Chemical Properties
Ytterbium(III) chloride (YbCl3) is a white powdery monoclinic crystals. It reacts with NiCl2 to form a very effective catalyst for the reductive dehalogenation of aryl halides. It is used in catalysis and as a NMR shift reagent in biology. It can also be used to track the digestion process in all animals.
Ytterbium(III) Chloride is a typical starting material for the production of Yb metal. It is also used a a source of Yb3+ ion for doping into laser host materials.
Uses
Used as laboratory reagent, optical glasses, structural ceramics, catalysts, electrical components and photo-optical material. Applied with NiCl2 to form a very effective catalyst for the reductive dehalogenation of aryl halides. Ytterbium(III) chloride is a powerful catalyst for the formation of acetals using trimethyl orthoformate and also used to track digestion in animals.
Definition
ChEBI: Ytterbium(III) chloride is the trichloride salt of ytterbium(III). It has a role as a NMR shift reagent. It contains a ytterbium(3+).
Preparation
Anhydrous ytterbium(III) chloride can be produced by the ammonium chloride route. In the first step, ytterbium oxide is heated with ammonium chloride to produce the ammonium salt of the pentachloride:
Yb2O3 + 10NH4Cl → 2(NH4)2YbCl5 + 6H2O + 6NH3
In the second step, the ammonium chloride salt is converted to the trichlorides by heating in a vacuum at 350-400°C:
(NH4)2YbCl5 → YbCl3 + 2HCl + 2NH3
reaction suitability
reagent type: catalyst
core: ytterbium
Safety Profile
Poison by
intraperitoneal route. Mtldly toxic by
ingestion. An experimental teratogen. A skin
and eye irritant. When heated to
SYN: YTTERBIUM TRICHLORIDE
decomposition it emits toxic fumes of Cl-.
See also YITERBIUM, RARE EARTHS,
and CHLORIDES.