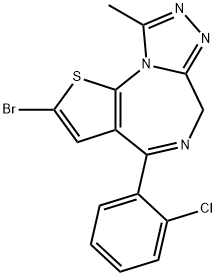
Description
Brotizolam is a hypnotic/sedative approximately 60-100 times
more potent than flurazepam. As with other short-acting, annulated
benzodiazepines (e.g., triazolam), cited advantages include
lack of adverse effects on sleep and performance after arousal.
Manufacturing Process
(a) 11.5 g of 7-bromo-5-(o-chlorophenyl)-3H-[2,3e]-thieno-1,4-diazepin-2-
one (see German Patent 2,221,623), were heated at 55° to 60°C with 100 cc
of absolute pyridine and 6.5 g of phosphorus pentasulfide for 4 hours while
stirring. The mixture was allowed to cool and was then poured into 100 cc of
saturated ice-cold NaCl solution. The precipitate was collected by suction
filtration, washed with water, dissolved in 100 cc of methylene chloride, the
solution was dried and evaporated, and the residue was treated with a little
methylene chloride. After suction filtration, 6 g of brown crystalline 7-bromo-
5-(o-chlorophenyl)-3H-[2,3e]-thieno-1,4-diazepine-2-thione, melting point
214°C (decomposition) were obtained.
(b) 6.0 g of this compound were suspended in 100 cc of tetrahydrofuran, and
the suspension was stirred at room temperature with 1.2 g of hydrazine
hydrate for 20 minutes. After evaporation to about 10 cc, 20 cc of ether were
added, and the crystals were collected by suction filtration. Yield: 5.2 g of 7-
bromo-5-(o-chlorophenyl)-2-hydrazino-3H-[2,3e]-thieno-1,4-diazepine,
melting point about 300°C (decomposition).
(c) 5.2 g of this compound were suspended in 50 cc of orthotriethyl acetate,
and the suspension was heated to 80°C. After about 30 minutes a clear
solution was first formed from which later colorless crystals separated out.
The mixture was allowed to cool, and the crystals were collected by suction
filtration and washed with ether. Yield: 5 g of the compound, melting point
211° to 213°C.