Description
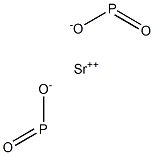
Strontium hypophosphite has the molecular formula
of Sr(H2PO2)2 and the molecular weight of 170.0631 g/
mol. This salt can be prepared by the aqueous reaction
of the carbonate or oxide with H3PO2:
SrO + 2H3PO20Sr(H2PO2)2
The salt is soluble in water (16 g/100 ml at 20°C)
so that the solution must be evaporated to obtain
crystals.
The structure of monoclinic strontium dihydrogenphosphinate,
Sr(H2PO2)2 consists of layers of hypophosphite
anions and metal cations exhibiting square antiprismatic coordination by O atoms. The Sr atoms are located on sites with point symmetry 21. Within the
layers, each anion bridges four metal cations.