Description
Polymers are frequently rendered flame resistive by
adding phosphorus and halogen compounds or
mixtures thereof to them. Some polymers are processed
at high temperatures, for example at 250°C or higher.
For this reason, many known flame retardants are
unsuitable for such applications, since they are too volatile
or are not sufficiently heat resistant.
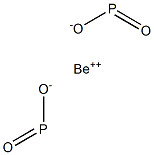
Beryllium forms a great number of organo-phosphinates
which have been studied extensively. Suchmaterials
have potential as a catalyst in controlling the growth and
size of polymers in general and in the production of fireresistant
polymers by incorporation of a metallic cation
like Be
2+.An example is the preparation andmeasurement
of the physical properties of suchmaterials using infrared,
DTA, and TGA methods of analysis.
The general structure of these organo-phosphinates has
been proposed to be a linear but three-dimensional cyclic
structure of phosphinate groups to which “R groups? dimethyl,
tetramethylene, di-n-butyl, etc.” are attached.