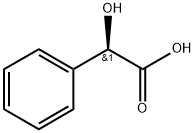
Chemical Properties
white to light yellow crystal powde
Uses
(
R)-(?)-Mandelic acid can be used:
- As a chiral acid in the separation of diastereomeric salts for the synthesis of chiral pyridoindole based building block.
- In the study of chiral acid selectivity of poly(3,4-ethylenedioxythiophene) (PEDOT) based chiral conducting?polymers.
Uses
(R)-(-)-Mandelic acid, is used as a antiseptic ingredient particularly against urinary tract infections.Mandelic acid and its derivatives are used to apply the dual activities as an antibacterial agent and as an antiaging agent. It is used as an intermediate for the synthesis of target molecules for other applications.
Uses
Antiseptic (urinary).
Definition
ChEBI: (R)-mandelic acid is the (R)-enantiomer of mandelic acid. It has a role as a human xenobiotic metabolite. It is a conjugate acid of a (R)-mandelate. It is an enantiomer of a (S)-mandelic acid.
General Description
(
R)-(-)-Mandelic acid, a chiral resolving agent, is also used as a building block to synthesize pharmaceutical drugs such as penicillin and cephalosporin. It can be synthesized from (
R,
S)-mandelonitrile with high yield and enantioselectivity using nitrilase enzyme.
Flammability and Explosibility
Non flammable
Purification Methods
Purify the mandelic acids by recrystallisation from H2O, *C6H6 or CHCl3. [Roger J Chem Soc 2168 1932,Jamison & Turner J Chem Soc 611 1942.] They have solubilities in H2O of ca 11% at 25o. [Banks & Davies J Chem Soc 73 1938.] The S-benzylisothiuronium salts has m 180o (from H2O) and [] D 25 (+) and (-) 57o (c20, EtOH) [El Masri et al. Biochem J 68 199 1958]. [Beilstein 10 IV 564.]