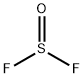
Chemical Properties
colourless gas
Physical properties
A colourless gas, thermally stable up to red heat. Does not corrode Fe, Ni, Co, Hg, Si, Mn, B, Mg, Al or Zn below 125°C. Does not attack glass. Suffocating odour. Hydrolyzed very slowly in ice-cold water. M.p. -110.5°C, b.p. -43.7°C, tcr +88°C; d.(liq.)(—100°C) 1.780, d. (solid) (-183°C) 2.095.
Preparation
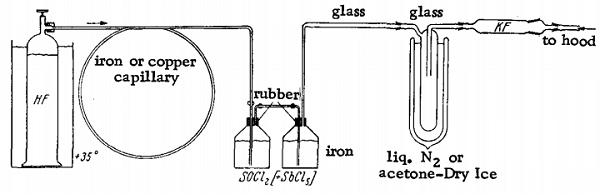
An iron bottle with a gas inlet tube serves as the reaction vessel. A second bottle is connected to the first to retain the unreacted HF. This is joined to a glass gas trap immersed in liquid nitrogen or a Dry Ice-acetone bath. A drying tube filled with KF is attached to exclude atmospheric moisture. The reaction vessel is filled with 500 g. of SOCl2 and 50 g of (catalyst), and anhydrous gaseous HF is introduced through the inlet tube. The HF is thoroughly absorbed, causing a mixture of SOF2 and HCl to evolve, which then is collected in the condensation trap. The reaction is so endothermic that the outside of the reaction vessel gradually becomes covered with ice. When all the SOCl2 has been consumed, more may be added without further SbCl5. Separation of the SOF2 from the HCl may be achieved either by distillation or by rapid bubbling of the gas mixture through ice-cold water, in which HCl is completely absorbed. At the same time, the SOF2 passes through with almost no decomposition. The gas is then dried over concentrated H2SO4 or over P2O5. Thionyl fluoride is stored under pressure in steel cylinders.
SOCl2 + 2 HF = SOF2 + 2 HCl
Biological Activity
Periostin (POSTN) participates in collagen fibrillogenesis. It plays a major role in the development of scar after traumatic spinal cord injury (SCI). Periostin controls the activities of fibrocyte to stimulate myofibroblast differentiation and lung fibrosis. It modulates the amount of collagen in tissue. Periostin also participates in the assembly of the extracellular matrix (ECM) architecture.
Safety Profile
Moderately toxic by
inhalation. A severe irritant to skin, eyes,
and mucous membranes. When heated to
decomposition or on contact with water or
steam it emits highly toxic and corrosive
fumes of SOx and F-. See also
FLUORIDES.