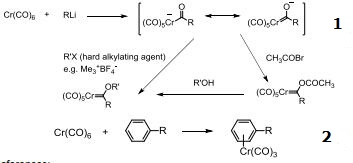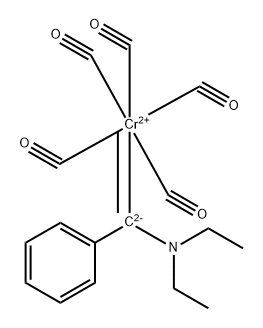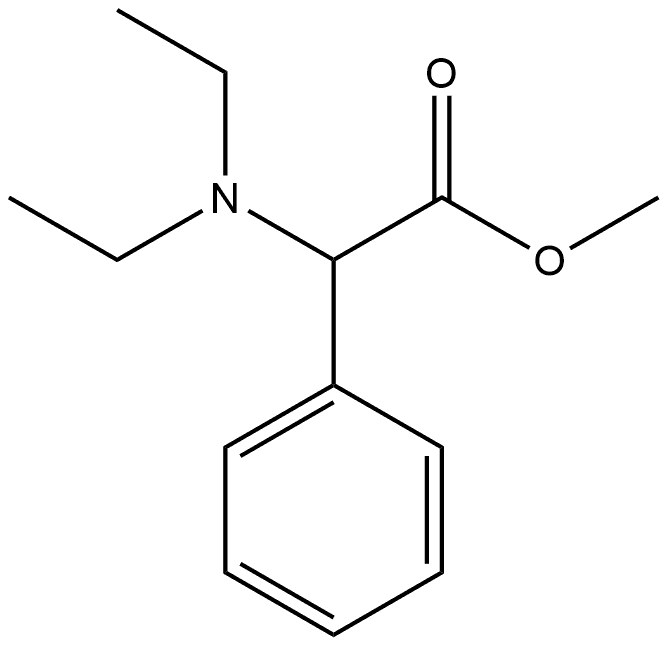
Chromium hexacarbonyl
- Product NameChromium hexacarbonyl
- CAS13007-92-6
- CBNumberCB9266096
- MF6CO.Cr
- MW220.06
- EINECS235-852-4
- MDL NumberMFCD00010945
- MOL File13007-92-6.mol
- MSDS FileSDS
Chemical Properties
| Melting point | >150 °C (dec.) (lit.) |
| Boiling point | 220 °C |
| Density | 1.77 g/mL at 25 °C (lit.) |
| vapor density | 7.6 (vs air) |
| vapor pressure | 1 mm Hg ( 36 °C) |
| storage temp. | Store below +30°C. |
| solubility | insoluble in H2O, ethanol; soluble in ethyl ether,chloroform |
| form | Crystals |
| Specific Gravity | 1.77 |
| color | White |
| Water Solubility | insoluble |
| Sensitive | Light Sensitive/Heat Sensitive |
| Merck | 13,2253 |
| Exposure limits | TLV-TWA: 0.05 mg (Cr)/m3, confirmed
human carcinogen (ACGIH) PEL: 0.1 mg (CrO3)/m3 (ceiling) (OSHA). |
| Stability | Stable. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 13007-92-6(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| FDA UNII | 920F3EJX2P |
Safety
| Symbol(GHS) |

|
|||||||||
| Signal word | Danger | |||||||||
| Hazard statements | H301 | |||||||||
| Precautionary statements | P264-P270-P301+P310-P405-P501 | |||||||||
| Hazard Codes | T,N,Xn | |||||||||
| Risk Statements | 22-50/53-49-44-43-20/21/22-5 | |||||||||
| Safety Statements | 53-36-45-61-60-36/37 | |||||||||
| RIDADR | UN 3466 6.1/PG 3 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | GB5075000 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 6.1 | |||||||||
| PackingGroup | II | |||||||||
| HS Code | 29310095 | |||||||||
| Toxicity | LD50 i.v. in mice: 100 mg/kg (Strohmeier) | |||||||||
| NFPA 704: |
|