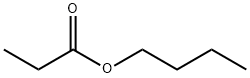
Description
Butyl propionate is a flammable, colorless tostraw-yellow liquid with an apple-like odor. Molecularweight = 130.2; Boiling point = 146℃; Specific gravity(H2O:1) = 0.87; Melting/Freezing point = - 90℃; Vaporpressure = 2.9 mmHg at 20℃; Relative density at 20℃ ofthe saturated vapor/air mixture (air = 1) = 1.01; Flashpoint = 32℃; Autoignition temperature = 426℃. HazardIdentification (based on NFPA-704 M Rating System):Health 2, Flammability 3, Reactivity 0. Practically insolublein water.
Chemical Properties
Butyl propionate is a flammable, colorless to
straw-yellow liquid with an apple-like odor.
Chemical Properties
Butyl propionate has a characteristic earthy, faintly sweet odor and apricot-like taste.
Occurrence
Reported found in fresh apple, apple juice, melon, strawberry, Gruyere de Comte cheese and plum.
Uses
Butyl propionate is a moderately fast evaporating solvent. Its linear structure contributes to effective viscosity reduction and improves solvent diffusion from coating films.
Butyl propionate is used solvent for nitrocellulose, retarder in lacquer thinner, ingredient of perfumes, flavors.
Preparation
By esterification of propionic acid with n-butyl alcohol in the presence of concentrated H2SO4 or p-toluene sulfonic acid.
Definition
ChEBI: Butyl propionate is a propanoate ester of butan-1-ol. It has a role as a plant metabolite, a human metabolite, an insect attractant and a flavouring agent. It is functionally related to a butan-1-ol.
Aroma threshold values
Detection: 25 to 440 ppb
General Description
A water-white liquid with an apple-like odor. Less dense than water. Flash point 90°F. May irritate skin and eyes. Used to make perfumes and flavorings.
Air & Water Reactions
Highly flammable. Very slightly soluble in water.
Reactivity Profile
BUTYL PROPIONATE is an ester. Esters react with acids to liberate heat along with alcohols and acids. Strong oxidizing acids may cause a vigorous reaction that is sufficiently exothermic to ignite the reaction products. Heat is also generated by the interaction of esters with caustic solutions. Flammable hydrogen is generated by mixing esters with alkali metals and hydrides.
Hazard
Skin and eye irritant. Flammable, moderate
fire risk.
Health Hazard
Contact will cause eye and skin irritation. Vapor exposure may cause eye and respiratory tract irritation.
Fire Hazard
Special Hazards of Combustion Products: Irritating and toxic gases, such as carbon dioxide and carbon monoxide, may be produced in fire.
Flammability and Explosibility
Flammable
Safety Profile
Mildly toxic by
ingestion. A skin irritant. Dangerously
flammable when exposed to heat or flame.
To fight fire, use foam, CO2, dry chemical.
Incompatible with oxidizing materials. See
also ESTERS, n-BUTYL ALCOHOL, and
PROPIONIC ACID.
Potential Exposure
It is used as a solvent or lacquer thinner; and in perfumes and flavoring
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit.
storage
Color Code—Red: Flammability Hazard: Storein a flammable liquid storage area or approved cabinetaway from ignition sources and corrosive and reactivematerials. Protect against physical damage. Outside ordetached storage is preferred. Prior to working with butylpropionate you should be trained on its proper handlingand storage. Store in tightly closed containers in a cool,well-ventilated area away from incompatible materialslisted above. Metal containers involving the transfer ofthis chemical should be grounded and bonded. Drumsmust be equipped with self-closing valves, pressure vacuum bungs, and flame arresters. Use only nonsparkingtools and equipment, especially when opening and closingcontainers of this chemical. Sources of ignition, such assmoking and open flames, are prohibited where this chemical is used, handled, or stored in a manner that could create a potential fire or explosion hazard
Shipping
UN1914 Butyl propionates, Hazard Class: 3;
Labels: 3—Flammable liquid
Incompatibilities
Vapor may form explosive mixture with
air. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep
away from alkaline materials, strong bases, strong acids,
oxoacids, epoxides. May accumulate static electrical
charges and cause ignition of its vapors.
Toxics Screening Level
The initial threshold screening level (ITSL) for n-butyl propionate is 102 μg/m3 (annual averaging time).
Waste Disposal
Dissolve or mix the material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. All federal,
state, and local environmental regulations must be
observed.