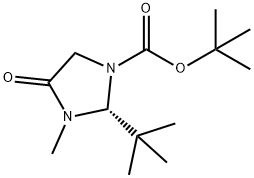
Synthesis
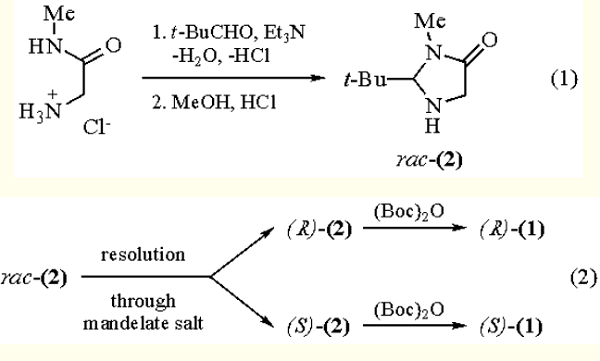
The commercial glycine N-methylamide hydrochloride is converted to the racemic imidazolidinone (2) by imine formation with Pivalaldehyde and cyclization under acidic conditions (eq 1). The mandelate salt of like configuration is less soluble and is used for highly efficient resolution; subsequent treatment with Boc anhydride (Di-t-butyl Dicarbonate) gives the enantiomeric Boc-BMI (1) (eq 2).
References
1. Fitzi, R.; Seebach, D. T 1988, 5277.
2. Seebach, D.; Fitzi, R. Ger. Patent 3 604 591, 1986 (CA 1988, 108, 94 944j).
3. Seebach, D.; Dziadulewicz, E.; Behrendt, L.; Cantoreggi, S.; Fitzi, R. LA 1989, 1215.