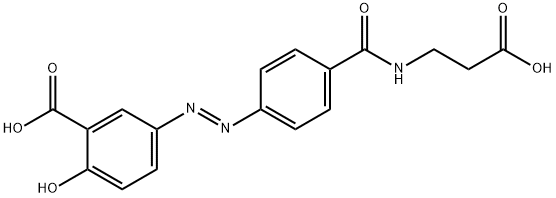
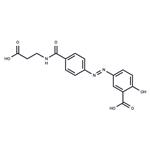
Description
Colazide was launched in the UK for mild to moderate acute attacks of
ulcerative colitis. It can be prepared by diazotization of 3-(4-aminobenzoylamino)
propionic acid followed by condensation with salicylic acid. Colazide, an
analogue of sulfasalazine, was found to be non-toxic and non-mutagenic in the Ames
test. Once ingested the molecule releases a non-toxic fragment, 4-aminobenzoyl-β-alanine
(poorly absorbed - 75% excretion in stool), and the active component 5-
aminosalicylic acid (Mesalazine). The exact mechanism of action is not clearly
understood but it is cytoprotective and has antiinflammatory properties. Other
biological effects have been observed and include the following: (a) granulocyte
activation is blocked, (b) there is a reduction in myeloperoxidase activity, and (c) a
reduction in the release of arachidonic acid with a concomitant decrease in
prostaglandin and leucotriene (as indicated by the lack of production of LTB
4)
production. It can behave as a reactive oxygen species (ROS) scavenger, inhibit PAF
formation, reduce IL-1 production, and antagonize TNF and NK cells. While
comparable in efficacy to sulfasalazine, Colazide removes the possibility of
sulfasalazine side-effects, such as, agranulocytosis, hepatotoxicity and male infertility.
Description
Balsalazide is a prodrug form of 5-aminosalicylic acid (5-ASA; ). It is cleaved by bacterial azoreductases in the intestinal lumen to release 5-ASA. Balsalazide (600 mg/kg per day) decreases IL-2 levels and increases IL-6 levels in the serum and colonic mucosa membrane, as well as decreases micro- and macroscopic colonic damage, in a rat model of colitis induced by 2,4-dinitrochlorobenzene (DNCB). Formulations containing balsalazide have been used in the treatment of ulcerative colitis.
Chemical Properties
Yellow Solid
Uses
Balsalazide-d4 is an isotopically labelled analog of Balsalazide (B116300), which is an analogue of Sulfasalazine (S699084). A prodrug of 5-aminosalicylic acid where carrier molecule is 4-aminobenzoyl-β-alanine. Anti-inflammatory (gastrointestinal).
Uses
An analogue of Sulfasalazine, A prodrug of 5-Aminosalicylic Acid
Definition
ChEBI: A monohydroxybenzoic acid consisting of 5-aminosalicylic acid (mesalazine) linked to 4-aminobenzoyl-beta-alanine via an azo bond.
Manufacturing Process
125 g finely powdered 4-nitrobenzoyl chloride were added portionwise, while
stirring, to a solution of 70 g β-alanine in 500 ml water containing 65 g
sodium hydroxide and cooled to 5°C. The reaction mixture was stirred for 3
hours and then added to a mixture of ice and hydrochloric acid. The
precipitate obtained was filtered off, washed with water and dried by suction.
After crystallisation of the dried product from hot acetone, there were
obtained 130 g 4-nitrobenzoyl-β-alanine, M.P. 164°-166°C.
A suspension of 15 g finely powdered 4-nitrobenzoyl-β-alanine in 200 ml
ethanol was stirred in an atmosphere of hydrogen in the presence of 1 g of
palladium-charcoal (5%), while cooling gently. When the absorption of
hydrogen had ceased, the reaction mixture was filtered and the filtrate
concentrated to a small volume. Upon adding diethyl ether and cooling 4-
aminobenzoyl-β-alanine was obtained. The yield was 11.5 g, M.P. 156°-158°C.
8.8 g 4-aminobenzoyl-β-alanine were triturated with 12 ml hydrochloric acid
and the paste obtained was dissolved in 100 ml water. The solution was
cooled to -5°C and a solution of 3 g sodium nitrite in 20 ml water, cooled to
0°C, was added dropwise, while stirring. The diazotised solution was left for 1
hour at 0°C and was then added dropwise at -5°C to a solution of 6 g salicylic
acid in 70 ml water containing 3.6 g sodium hydroxide and 7 g sodium
carbonate. The final reaction mixture was adjusted to a pH of about 8, stirred
for 2 to 3 hours and added to a mixture of dilute hydrochloric acid and ice.
The precipitate obtained was filtered off, washed with water and suction dried.
Crystallisation from hot ethanol gave 11.9 g 5-[(2-carboxy-ethylcarbamoyl)-
phenylazo]-2-hydroxy-benzoic acid, M.P. 254°-255°C.
10.7 g of the free acid were dissolved in 300 ml warm ethanol and treated
with a solution of 2.4 g sodium hydroxide in 25 ml ethanol. The precipitate
obtained was filtered off, washed with ethanol and diethyl ether and dried in a
vacuum at 50°C to give 11.5 g of the disodium salt of 5-[(2-carboxyethylcarbamoyl)-
phenylazo]-2-hydroxy-benzoic acid, M.P. >350°C.
brand name
Colazal
(Salix).
Therapeutic Function
Antiinflammatory
Clinical Use
Treatment and maintenance of remission, in mild to
moderate ulcerative colitis
Metabolism
Very little of an oral dose of balsalazide is absorbed
via the upper gastrointestinal tract, and almost the
entire dose reaches its site of action in the colon intact.
It is broken down by the colonic bacterial flora into
5-aminosalicylic acid (mesalazine), which is active, and
4-aminobenzoylalanine, which is considered to be an
inert carrier. Most of a dose is eliminated via the faeces,
but about 25% of the released mesalazine is absorbed and
acetylated. A small proportion of 4-aminobenzoylalanine
is absorbed and acetylated by first-pass metabolism
through the liver. The acetylated metabolites are excreted
in the urine.