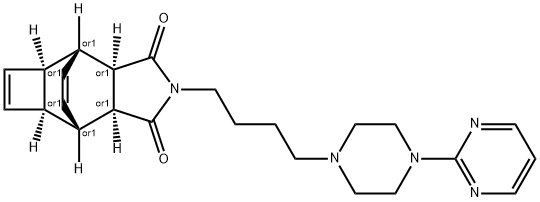
Manufacturing Process
To a stirred solution of 0.035 mole of 1,3-dioxo-4,7-etheno-δ 5 -1,3,3a,7a-
tetrahydrocyclobut[f]isoindole in 70 ml of dimethylformamide is added 0.9 g
of sodium hydride. The suspension is stirred at 60°C for 3 hours and is poured
into a stirred solution of 1,4-dibromobutane (0.04 mol) in 50 ml of
dimethylformamide. The reaction mixture is stirred at room temperature for
24 hours, dimethylformamide is evaporated under reduced pressure and the
residue is extracted with methylene chloride (3x200 ml). The methylene
chloride extracts are collected, washed with water, dried over anhydrous
Na 2 SO 4 and evaporated under reduced pressure. The residue is solidified to a
waxy like material. 0.007 mole of this material is dissolved in 50 ml of
dimethylformamide, and to this solution is added 6 ml of triethylamine and
0.007 mole of 1-(2-pyrimidinyl)piperazine dihydrochloride. The reaction
mixture is stirred at room temperature for 48 hours. Dimethylformamide is
removed under reduced pressure and the remaining solid is extracted with
2x100 ml of methylene chloride. The methylene chloride extracts are dried
over anhydrous Na 2 SO 4 , evaporated and the residue is separated by HPLC
using 30% methanolethyl acetate as eluent. Evaporation of the solvent from
the desired fractions (R f = 0.5) affords the 3a,4,4a,6a,7,7a-hexahydro-2-[4-
[4-(2-pyrimidinyl)-1-piperazinyl]butyl]-4,7-etheno-1H-cyclobut[f]isoindole-
1,3(2H)-dione dihydrochloride, which is converted to the dihydrochloride salt
by dissolving the free base in ethanol and adding ether saturated with
hydrogen chloride; MP: 252°-254°C.