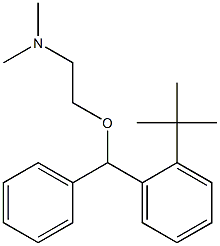
Manufacturing Process
The ο-t-butyl-α-phenylbenzyl alcohol and N,N-dimethylmonochloroacetamide
are dissolved in anhydrous diethyl ether. Portionwise 50% sodium hydride in
the form of an oily suspension is added to the solution with stirring. After all
the sodium hydride is added, the mixture is stirred at room temperature for
another 3 h and then water is added to decompose excess sodium hydride.
The ethereal layer is separated, dried with sodium sulfate, filtered and
concentrated by removal of the solvent. The residue crystallizes upon addition
of petroleum ether (boiling range 80-100°C) to which some diethyl ether is
added. There is obtained 2-[ο-t-butyl-α-phenylbenzyl)oxy]-N,N-dimethyl
acetamide, melting point 90-91°C (81% yield).
To a solution of 2-[ο-t-butyl-α-phenylbenzyl)oxy]-N,N-dimethyl acetamide in
anhydrous diethyl ether is added portionwise with stirring and while refiuxing
lithium aluminum hydride. After the addition is completed, refluxing is
continued overnight. The reaction mixture still contains some lithium
aluminum hydride which is decomposed by the addition of water. The mixture
is filtered, the ethereal solution separated and dried over sodium sulfate.
Diethyl ether is removed from the ethereal solution by distillation. There is
obtained 2-[(ο-t-butyl-α-phenylbenzyl)oxy]-N,N-dimethylethylamine.