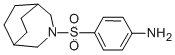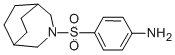Manufacturing Process
The 1,4-cyclohexane-bis(methylamine) feed line was connected to the top of
the preheater in a pyrolysis tube. The pyrolysis section was heated by
external electric heaters. For a typical run, the pyrolysis temperature was maintained at 385°-395°C, the feed rate was 8.4 g per minute of 1,4-
cyclohexanebis(methylamine) and 1.47 g per minute of nitrogen this is a ratio
of 0.885 mole of nitrogen per mole of 1,4-cyclohexanebis(methylamine) per h.
The crude reaction product was collected in a chilled flask and the nitrogen
and reaction gases were vented to a hood. These conditions were maintained
for 15.5 h, during which time 7,849 g (55 moles) of 1,4-
cyclohexanebis(methylamine) was fed to the unit. Tile total crude product
obtained weighed 7,269 g. Distillation of the crude product at atmospheric
pressure to a base temperature of 260°-270°C yielded 1,480 g of 3-
azabicyclo[3.2.2]nonane (recrystallized from an equal weight of acetone). Gas
chromatography of the filtrates of this first fraction through a 6-foot column
packed with 15% Carbowax 20M on white Chromosorb indicated 1,854 g of
1,4-cyclohexanebis(metnylamine) and 721.0 g of 3-azabicyclo[3.2.2]nonane
present. The remainder of the crude product was distilled at 1-5 mm to a
base temperature of 200°-225°C. Thus, a total of 2,201 g (17.6 moles, 62.1%
yield) of 3-azabicycla[3.2.2]nonane was produced.
To a 3 L, three-neck flask equipped with a stirrer, thremometer, and condenser
was charge 30.0 g (0.24 mole) of 3-azabicyclo[3.2.2]nonane, 44.3 g (0.2
mole) of p-nitrobenzenesulfonyl chloride and 2 L of water. The pH of the
reaction mixture was adjusted to 14 with a 10% solution of sodium hydroxide,
the reaction mixture was then slowly heated to 75°C and heating stopped.
The reaction mixture was cooled to 20°C and 44.0 g (71% of theory) of crude
3-(p-nitrobenzenesulfonyl)-3-azabicyclo[3.2.2]nonane was collected by
filtration.
The crude 3-(p-nitrobenzenesulfonyl)-3-azabicyclo[3.2.2]nonane (44.0 g, 0.14
mole), 5.0 g alcohol wet Raney nickel, and 400 ml methyl alcohol were
charged to an autoclave and reduced at 70°C at 1000 p.s.i. hydrogen
pressure until absorption of hydrogen had stopped. The crude reduced product
was filtered hot to remove the Raney nickel catalyst. The filtrate was cooled to
20°C and the solid 3-(p-ammobenzenesulfonyl)-3-azabicyclo[3.2.2]nonane
was collected by filtration (38.9 g 98% theory), melting point 149°-151°C.