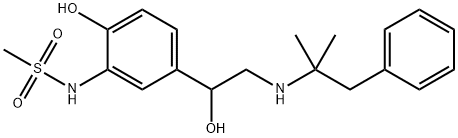
Uses
Zinterol is a β2-adrenergic agonist. It can be used to induce smooth muscle relaxation in the lungs, gastrointestinal tract, uterus, and various blood vessels.
Manufacturing Process
A). 2'-Hydroxy-5'-([N-(2-methyl-1-phenyl-2-propyl)glycyl]methane-
sulfonanilide hydrobromide:
To a solution of α,α-dimethylphenthylamine (120 g, 0.8 mole) in 1.1 liter of
acetonitrile is added 5'-bromoacetyl-2'-hydroxymethanesulfonanilide (108 g,
0.35 mole) in a period of 5 minutes. The resulting solution is refluxed for 5
minutes on a steam bath and then permitted to stand for 25 minutes at room
temperature after which it is chilled and acidified with 5 N ethanolic hydrogen
bromide. The acidified mixture is concentrated in vacuum until a thick slurry is
obtained. After standing overnight at room temperature, the slurry is filtered
and the crude product triturated with 2-butanone, filtered, washed with 2-
butanone and dried to 86.3 g, (54%), MP: 217.5-221°C (dec.).
B). 2'-Hydroxy-5'-(1-hydroxy-2-(2-methyl-1-phenyl-2-propylamino)ethyl)
methanesulfonanilide hydrobromide:
2'-Hydroxy-5'-([N-(2-methyl-1-phenyl-2-propyl)glycyl]methanesulfonanilide
hydrobromide (132 g, 0.29 mole is dissolved in 2 liters of hot methanol, the
methanolic solution is allowed to cool to room temperature and 13 g 10%
palladium on carbon catalyst suspended in 50 ml of water is added.
Hydrogenation of the stirred mixture is carried out under 1-3 atm. of pressure
for 17 hours during which time 0.31 mole of hydrogen is absorbed. The
catalyst is filtered and the filtrate concentrated under reduced pressure until a
thick slurry is obtained. Isoptopanpl is added tothed slurry and the mixture is
again concentrated in vacuum to remove water by azeotropic distillation.
Trituration of residual solid with 2-propanol and collection on a filter affords
100.5 g (76% yield) of desired product, MP: 194.5-195.5°C (dec.).
2'-Hydroxy-5'-(1-hydroxy-2-(2-methyl-1-phenyl-2-propylamino)ethyl)
methanesulfonanilide base:
2'-Hydroxy-5'-(1-hydroxy-2-(2-methyl-1-phenyl-2-propylamino)ethyl)
methanesulfonanilide hydrobromide (47.7 g) is refluxed with 100 ml of
methanol. The material only partly dissolves. A solution of 6.5 g of potassium
hydroxide in 25 ml of methanol is then added to the suspension followed by 1
L of water. The mixture is thoroughly stirred and cooled to 5-10°C. The
precipitate is collected on a filter and washed with water until a negative test
for bromide using silver nitrate is obtained. The product is dried in an oven at
65°C, yield 36 g.