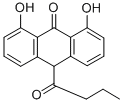
Manufacturing Process
To a solution of 56.6 g (0.25 mol) of anthralin in 1750 ml of absolute toluene
and 27.3 ml of pyridine, 31.5 ml (0.3 mol) of butyryl chloride was added with
stirring over 30 min at room temperature. The reaction mixture was then
heated to 85°C for 1 hour. After this mixture had recooled to room
temperature, 27.3 ml of pyridine and 31.5 ml of butyryl chloride were again
added. The suspension obtained was then heated to 85-90°C for 1 hour. The
precipitated pyridinium hydrochloride was eliminated by filtration then washed
with toluene. The toluene filtrates were concentrated to around 500 ml under
reduced pressure, washed several times with water then dried over
magnesium sulfate. The product was then fractionated by chromatography on silica gel, using toluene and then a mixture of toluene and ethyl acetate as
the mobile phase. The different fractions containing the 1,8-dihydroxy-10-(1-
oxobutyl)-9(10H)-anthracenone were then concentrated and recrystallized
from a toluene-hexane mixture. In this way 25 g of yellow crystals of 1,8-
dihydroxy-10-(1-oxobutyl)-9(10H)-anthracenone having a melting point of
138°C was obtained.