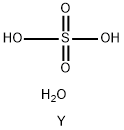
YTTRIUM SULFATE OCTAHYDRATE
- Product NameYTTRIUM SULFATE OCTAHYDRATE
- CAS7446-33-5
- CBNumberCB9105739
- MFH4O5SY
- MW204.99
- MDL NumberMFCD00149946
- MOL File7446-33-5.mol
Chemical Properties
| Density | 2.5 g/mL at 25 °C(lit.) |
| form | Crystalline |
| Specific Gravity | 2.558 |
| color | White to pale yellow |
| Water Solubility | Water solubility decreases with increasing temperature. Soluble in water. |
| Merck | 14,10107 |
| CAS DataBase Reference | 7446-33-5(CAS DataBase Reference) |
Safety
| Symbol(GHS) |

|
|||||||||
| Signal word | Warning | |||||||||
| Hazard statements | H315-H319-H335 | |||||||||
| Precautionary statements | P280a-P304+P340-P405-P501a-P261-P305+P351+P338 | |||||||||
| Hazard Codes | Xi | |||||||||
| Risk Statements | 36/37/38 | |||||||||
| Safety Statements | 26-37/39 | |||||||||
| WGK Germany | 3 | |||||||||
| TSCA | Yes | |||||||||
| NFPA 704: |
|