Originator
Kefglycin,Shionogi,Japan,1969
Manufacturing Process
dl-Phenylglycine is resolved in a conventional manner by reaction with
cinchonine, fractional crystallization of the resulting diastereoisomers, and
acidification to release the phenylglycine enantiomorphs. D-phenylglycine,
thus prepared, is reacted with carbobenzoxy chloride in a conventional
manner to produce N-carbobenzoxy-D-phenylglycine.
A 0.60 g portion of N-carbobenzoxy-D-phenylglycine is dissolved in 10 ml of
dry tetrahydrofuran. The solution is cooled in an ice-salt bath, and to it is
added 0.29 ml of triethylamine with stirring over a period of 10 minutes,
followed by 0.29 ml of isobutyl chloroformate, after which stirring is continued
for 10 minutes at -5°C. During this time, 0.57 g of 7-aminocephalosporanic
acid and 0.29 ml of triethylamine are dissolved in 5 ml of tetrahydrofuran and
5 ml of water, and the solution is centrifuged to remove a dark sludge. The
clarified solution is cooled in ice and slowly added to the reaction mixture, and
stirring is continued in the ice bath for 0.5 hour, followed by one hour at room
temperature.
The reaction product mixture is a homogenous solution having a pH of about
6. It is evaporated under vacuum to a semisolid residue. To the residue are
added 35 ml of water and a few drops of triethylamine to raise the pH to 8.
The aqueous solution obtained thereby is extracted successively with 50 ml
and 35 ml portions of ethyl acetate, the pH being adjusted to 2 at each
extraction with hydrochloric acid. The extracts are combined, filtered, dried
over sodium sulfate, stripped of solvent, and evaporated under vacuum. The
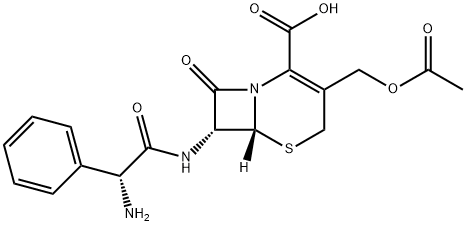
product is 7-(N-carbobenzoxy-D-α-aminophenylacetamido)cephalosporanic
acid in the form of a yellow-white amorphous solid weighing 1.10 g.
Of this material 1.0 g is dissolved in 150 ml of warm 95% ethyl alcohol. To
the solution is added 1.0 g of 5% palladium on carbon catalyst, and the
mixture is hydrogenated at room temperature and atmospheric pressure by
bubbling hydrogen into it for 3 hours with stirring. The hydrogenation product
is filtered. The solid phase, comprising the catalyst and the desired product, is
suspended in ethyl acetate and water and adjusted to pH 2 with hydrochloric
acid. The suspension is filtered to remove the catalyst. The aqueous phase is
separated from the filtrate, and is evaporated under vacuum to recover the
desired product, 7-(D-α-aminophenylacetamido)cephalosporanicacid.