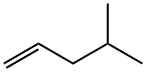
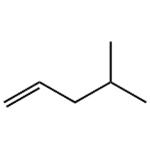
Chemical Properties
clear colourless liquid
Uses
Organic synthesis, monomer for plastics used inautomobiles, electronic components, and laboratoryware.
Uses
4-Methyl-1-pentene is used as a monomer for olefin polymerisation. The resulting polymer is poly(4-methyl-1-pentene). It can also be used to produce 1,2-diiodo-4-methyl-pentane.
General Description
Colorless liquid.
Air & Water Reactions
Highly flammable. Water insoluble.
Reactivity Profile
The unsaturated aliphatic hydrocarbons, such as 4-Methyl-1-pentene, are generally much more reactive than the alkanes. Strong oxidizers may react vigorously with them. Reducing agents can react exothermically to release gaseous hydrogen. In the presence of various catalysts (such as acids) or initiators, compounds in this class can undergo very exothermic addition polymerization reactions.
Hazard
Same as for 2-methyl-1-pentene.
Health Hazard
Harmful if inhaled or swallowed. Vapor or mist is irritating to the eyes, mucous membrane and upper respiratory tract. Causes skin irritation. Symptoms of exposure may include burning sensation, coughing, wheezing, laryngitis, shortness of breath, headache, nausea and vomiting.
Fire Hazard
Special Hazards of Combustion Products: Vapors may travel considerable distance to source of ignition and flashback. Container explosion may occur under fire conditions. Forms explosive mixtures in air.
Source
California Phase II reformulated gasoline contained 4-methyl-1-pentene at a
concentration of 300 mg/kg (Schauer et al., 2002).
Environmental Fate
Photolytic. Atkinson and Carter (1984) reported a rate constant of 1.06 x 10
-16 cm
3/molecule?sec
for the reaction of 4-methyl-1-pentene in the atmosphere.
Chemical/Physical. Complete combustion in air yields carbon dioxide and water.