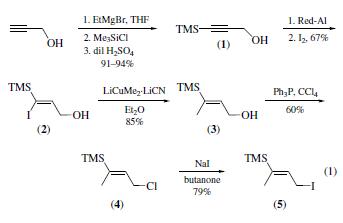
Preparation
the first reported preparation of this
reagent lacked full experimental details. The preparation given is a compilation of thework of several groups; the various
steps have been selected to give optimum yields. 2-Propyn-1-ol
is converted3 into its 3-(trimethylsilyl) derivative (1), which is
then reduced4 using sodium bis(2-methoxyethoxy)aluminum hydride (Red-Al). The intermediate vinylaluminum is reacted
with iodine to produce the (Z)-iodoalkene (2). Treatment of this
with lithium dimethylcuprate gives the allylic alcohol (3);.
transformation into (E)-(3-chloro-1-methyl-1-propenyl)trimethylsilane
(4) followed by halogen exchange with sodium
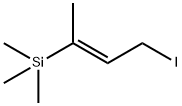
iodide then gives reagent (5).