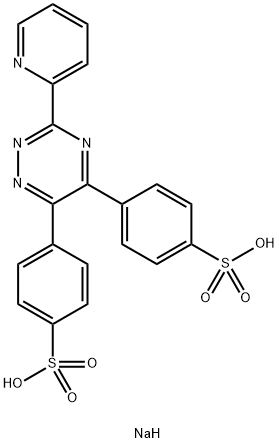
Physical properties
The visible absorption spectrum of the ferrous complex of ferrozine exhibits a single sharp peak with maximum absorbance at 562 nm. At this wavelength, the molar absorptivity is 27,900 and the Beer-Lambert law is obeyed to approximately 4 mg/liter of Fe.
It has been determined by spectrophotometric titration that ferrozine forms the expected tris complex with ferrous iron. The magenta Fe(ligand)8z+ species will form completely in aqueous solution between the pH values of 4 and 9. Once formed between these values, the complex is stable to 1N perchloric acid.