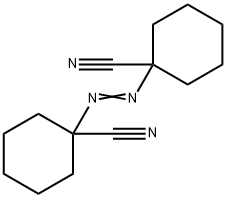
1,1'-Azobis(cyanocyclohexane)
- Product Name1,1'-Azobis(cyanocyclohexane)
- CAS2094-98-6
- CBNumberCB8687740
- MFC14H20N4
- MW244.34
- EINECS218-254-8
- MDL NumberMFCD00046334
- MOL File2094-98-6.mol
- MSDS FileSDS
Chemical Properties
| Melting point | 114-118 °C(lit.) |
| Boiling point | 423.9±38.0 °C(Predicted) |
| Density | 1.13±0.1 g/cm3(Predicted) |
| vapor pressure | 0-0.001Pa at 20-25℃ |
| storage temp. | 2-8°C |
| form | Solid:crystalline |
| BRN | 960744 |
| Stability | Stable, but decomposes exothermically above about 114 C |
| InChI | 1S/C14H20N4/c15-11-13(7-3-1-4-8-13)17-18-14(12-16)9-5-2-6-10-14/h1-10H2/b18-17+ |
| InChIKey | KYIKRXIYLAGAKQ-ISLYRVAYSA-N |
| SMILES | N#CC1(CCCCC1)\N=N\C2(CCCCC2)C#N |
| LogP | 3.256 at 25℃ and pH5-6 |
| CAS DataBase Reference | 2094-98-6(CAS DataBase Reference) |
| FDA UNII | 9Y0B93KKUS |
| EPA Substance Registry System | 1,1'-Azobiscyclohexanecarbonitrile (2094-98-6) |
| UNSPSC Code | 12162002 |
| NACRES | NA.23 |
Safety
| Symbol(GHS) |
 
|
| Signal word | Danger |
| Hazard statements | H242-H315-H319-H335 |
| Precautionary statements | P210-P235-P302+P352-P305+P351+P338-P370+P378-P403 |
| Hazard Codes | F,Xi |
| Risk Statements | 11-36/37/38 |
| Safety Statements | 26 |
| RIDADR | UN 3226 4.1 |
| WGK Germany | 3 |
| F | 4.9 |
| TSCA | TSCA listed |
| HazardClass | 4.1 |
| PackingGroup | II |
| Storage Class | 5.2 - Organic peroxides and self-reacting hazardous materials |
| Hazard Classifications | Eye Irrit. 2 Self-react. D Skin Irrit. 2 STOT SE 3 |

