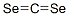Chemical Properties
light sensitive, golden yellow, strongly refractive liquid; odor of rotten radishes; turns brown to black on storage [MER06]
Preparation
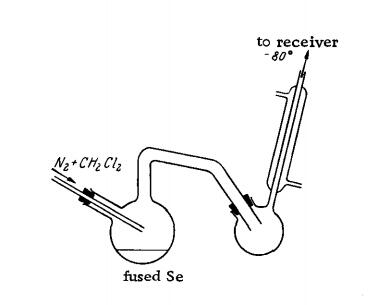
The preparation method of carbon selenide: a dry stream of N2 is saturated with CH2Cl2 vapor, and the gas mixture is introduced into a Vycor flask in which Se is heated to 550-600°C. The crude product precipitates in the receiver, equipped with a Liebig condenser. The cooling by the condenser is stopped and the liquid is forced with steam into a cooled flask, separated from the H2O and dried over CaCl2. It is then distilled at 46°C and 50 mm. in a fractionating column (30 cm. long).
Solubility in organics
Dissolves, yielding a yellow liquid, in CS2, CCl4, ether, benzene, nitrobenzene, dioxane, ethyl acetate and acetone. Slightly soluble in glacial acetic acid and alcohol, decomposing these rapidly; pyridine behaves in the same manner. Dissolves copious quantities of flowers of sulfur, but red Se hardly at all.