Description
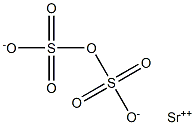
Strontium pyrosulfate has the molecular formula of
SrS
2O
7 and the molecular weight of 263.7478 g/mol. It
is expected to be more stable to decomposition in water
than the Be, Mg and Ca homologues but no specific data
on this aspect has appeared in the literature. There are
no references in the scientific literature concerning this
salt. Whether this salt is stable in aqueous solution
remains unknown. This may explain why the data on
strontium pyrosulfate (disulfate) is sparse to nonexistent.
It can be prepared via a solid-state reaction with
ammonium pyrosulfate:
SrCl2+(NH4)2S2O7+heat
? SrS2O7+2NH3(gas)+H2O(gas)
The solubility of this salt has not been reported. The
existence of hydrates is unknown. However, if one heats
the persulfate, a pyrosulfate salt can be obtained:
2SrS2O8 ? 2SrS2O7+O2
Further heating then forms the sulfate plus oxygen.
These reactions have not been studied critically and no
decomposition temperatures can be quoted. Another
method of preparation involves the sulfate. If strontium
sulfate is mixed with fuming sulfuric acid (to minimize
the water present), it dissolves forming a syrup which
upon heating to 150°C deposits the pyrosulfate crystals
which are stable. Further heating removes water and the
excess acid. These crystals are stable to about 720°C
where they decompose to form the sulfate plus sulfur
trioxide.