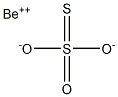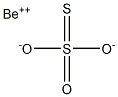Description
Beryllium thiosulfate would have the molecular
formula of [Be(OH)]
2S
2O
3 and the molecular weight of 155.1571 g/mol. It can be prepared by the reaction of
sodium thiosulfate upon the chloride:
BeCl2+Na2S2O3 ? [Be(OH)]2S2O3+2NaCl
Since this thiosulfate is expected to be soluble in
water, the solution must be evaporated at a low temperature
to crystallize the product [salt-out temperature
~15°F (—9.4°C)]. However, there is absolutely no
description in the scientific literature of the preparation
of this compound, either by the use of sodium thiosulfite
(basic solution) or the use of thiosulfuric acid (acidic
solution).
Beryllium thiosulfate is not commonly mentioned in
the literature or reference books. Only a few articles
prevail, mostly in patents, which discuss technically
specific physical uses along with many other similar
compounds.