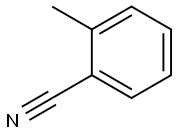
Chemical Properties
clear colorless to slightly yellow liquid
Uses
o-Tolyl Cyanide, is a versatile intermediate, used in the synthesis of various pharmaceutical and biologically active compounds. It can be used in the preparation of highly efficient triarylene conjugated dyes for sensitized solar cells
Synthesis Reference(s)
The Journal of Organic Chemistry, 60, p. 2948, 1995
DOI: 10.1021/jo00114a060
General Description
Light blue clear liquid.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
Nitriles, such as o-Tolunitrile, may polymerize in the presence of metals and some metal compounds. They are incompatible with acids; mixing nitriles with strong oxidizing acids can lead to extremely violent reactions. Nitriles are generally incompatible with other oxidizing agents such as peroxides and epoxides. The combination of bases and nitriles can produce hydrogen cyanide. Nitriles are hydrolyzed in both aqueous acid and base to give carboxylic acids (or salts of carboxylic acids). These reactions generate heat. Peroxides convert nitriles to amides. Nitriles can react vigorously with reducing agents. Acetonitrile and propionitrile are soluble in water, but nitriles higher than propionitrile have low aqueous solubility. They are also insoluble in aqueous acids.
Fire Hazard
o-Tolunitrile is combustible.
Purification Methods
Fractionally distil the nitrile, wash it with conc HCl or 50% H2SO4 at 60o until the smell of isonitrile has gone (this also removes any amines), then wash it with saturated NaHCO3 and dilute NaCl solutions, then dry it with K2CO3 and redistil it. [Beilstein 9 IV 1703.]