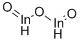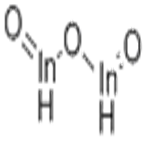General Description
Indium oxide (also known as indium sesquioxide, or In2O3) is a yellow colored powder. It is a stable ceramic-like material that is insoluble in water. Indium oxide is an n-type semiconductor and thus can be used as a resistive element in integrated circuits. It also is used to form heterojunctions with materials like p-InP, n-GaAs, n-Si, and other semiconductors.
Other applications include glass (as a color additive), alkaline batteries (to suppress gas formation), and high current electrical switches and contacts (as an anti-arcing additive).

Chemical Properties
light green powder
Physical properties
Light-yellow powder; cubic crystal; occurs in both amorphous and crys-talline forms; pale-yellow amorphous form converts to crystalline form onheating at higher temperatures; isomorphous with hematite, Fe
2O
3; density7.18 g/cm
3; melts around 2,000°C; insoluble in water; amorphous form dis-solves readily in mineral acids; crystalline form has low solubility in acids.
Uses
In glass manufacture.
Uses
Indium trioxide is used in specialty glass production.
Uses
Indium(III) oxide is widely utilized as a n-type semiconductor, which is used in integrated circuits as a resistive element. It finds applications in batteries and as a part of some stain formulations. It is used as thin film coatings in optical, antistatic and infrared reflectors. It is doped with tin oxide and used as transparent conductive coatings.
Preparation
Indium trioxide may be obtained by heating indium in air or oxygen:
4In + 3O
2 → 2In2O
3
or by calcination of indium hydroxide, nitrate, or carbonate at elevated tem-peratures:
2In(OH)
3 → In2O
3 + 3H
2O
In2(CO
3)
3 →In2O
3 + 3CO
2
General Description
In
2O
3/Au/Ag coated PET film
reaction suitability
reagent type: catalyst
core: indium
Purification Methods
Wash it with H2O and dry it below 850o. It volatilises at 850o and dissolves in hot mineral acids to form salts. Store it away from light because it darkens due to the formation of free In.